As a practitioner I never used to pay a lot of attention to Clinical Trial data. I assumed that if the FDA approved a drug, it meant that it was effective and that it added something to the drug armamentarium we already had. Back in the days when I was actively involved with treating psychotic patients [Schizophrenia], we had a collection of anti-psychotic drugs [now called the First Generation Antipsychotics] that all worked, but had somewhat different side effect profiles. I used the ones that had the least side effects [but they all had them]. I guess the rule was to find the lowest dose of the one the patient was most likely to take. They’re hard drugs to use. There’s a constant worry about side effects [particularly the irreversible one – Tardive Dyskinesia] and the effect of "drugging" the patient’s mind. On the other side of that coin is a crippling psychosis that once sentenced patients to an "Institutionalized" Life. It’s very easy to get stuck on the evils of one or the other side of that problem – to argue about being able to live freely in society, or to see drugs as a toxic chemical straight-jacket. For some, one doesn’t have to struggle and things go smoothly. But for a significant number of people with that illness, there’s no "right course" and one has to live in that netherworld as a Psychiatrist, just as the afflicted live there themselves. I’d been there before with the sometimes toxic treatments used in severe physical diseases [those dire warnings they mumble at the end of the t.v. drug commercials], but somehow the conflict feels different in Psychiatry, but that’s another very long subject for another day.
 Right now, I want to focus on the Atypical Antipsychotic drug Seroquel, one that’s often in the news, and in the stories about the intrusion of the Pharmaceutical Industry into the practice of Medicine [Psychiatry] over the last twenty or thirty years. The Atypical Antipsychotics are a group of drugs that are chemically different from the older drugs. When they began to appear, they were touted as having less extrapyramidal side effects [so less chance of irreversible Tardive Dyskinesia] and a diminished side effect profile in general. I had little to no experience with the drugs in my practice as a psychoanalytic psychotherapist, but in retirement I volunteer in clinics where I see some psychotic patients and a whole lot of people who have been started on these drugs elsewhere. The thing of it is, most of the people I see on Seroquel aren’t psychotic – nor have they ever been. Since its FDA approval, it was later approved for Mania [another psychotic illness] and then even later as an add-on medication for people with Major Depressive Disorder [psychotic or not] that don’t respond to anti-depressants [SSRIs]. In practice, it seems to be being used for depressed people
Right now, I want to focus on the Atypical Antipsychotic drug Seroquel, one that’s often in the news, and in the stories about the intrusion of the Pharmaceutical Industry into the practice of Medicine [Psychiatry] over the last twenty or thirty years. The Atypical Antipsychotics are a group of drugs that are chemically different from the older drugs. When they began to appear, they were touted as having less extrapyramidal side effects [so less chance of irreversible Tardive Dyskinesia] and a diminished side effect profile in general. I had little to no experience with the drugs in my practice as a psychoanalytic psychotherapist, but in retirement I volunteer in clinics where I see some psychotic patients and a whole lot of people who have been started on these drugs elsewhere. The thing of it is, most of the people I see on Seroquel aren’t psychotic – nor have they ever been. Since its FDA approval, it was later approved for Mania [another psychotic illness] and then even later as an add-on medication for people with Major Depressive Disorder [psychotic or not] that don’t respond to anti-depressants [SSRIs]. In practice, it seems to be being used for depressed people 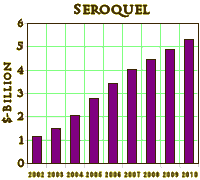 even if they don’t have "Major" depression, or people who have Anxiety, or people who have any number of other things including PTSD or insomnia of any kind. It’s being used a bit like the Benzodiazepines as an all purpose anxiolytic/sedative drug [we don’t use the Benzodiazepines much anymore except short-term because they tend to be habituating and addictive]. The staggering point is that the Atypical Antipsychotics are the number one drugs prescribed in the U.S. right now [by cost]. And Seroquel is the number one in the class – to the tune of $4.9 Billion last year [2009]! Cough.
even if they don’t have "Major" depression, or people who have Anxiety, or people who have any number of other things including PTSD or insomnia of any kind. It’s being used a bit like the Benzodiazepines as an all purpose anxiolytic/sedative drug [we don’t use the Benzodiazepines much anymore except short-term because they tend to be habituating and addictive]. The staggering point is that the Atypical Antipsychotics are the number one drugs prescribed in the U.S. right now [by cost]. And Seroquel is the number one in the class – to the tune of $4.9 Billion last year [2009]! Cough.
If you’re reading this, you can’t have missed that AstraZeneca and its Seroquel have been on the radar of the increasing throng of people up in arms about Pharmaceutical Companies using Physicians in stealth advertising of their drugs, particularly in Psychiatry – paid Speaker’s Bureaus, Ghost-writing, Scientific Advisory Boards, Continuing Medical Education, unacknowledged Conflicts of Interest, direct relationships with Key Opinion Leaders and Academic Psychiatrists. In 2008, Senator Chuck Grassley’s investigations and the numerous suits against Pharmaceutical Companies belatedly put some of these practices on the front pages of our newspapers. I’ve been writing about these things a lot myself, including some things about Seroquel.
Recently, Stephany [soulful sepulcher] sent me the URL of a key to a sea of documents released in May 2009 from one of the cases against AstraZeneca [a suit about covering up the incidence of weight gain and Diabetes as a side effect]. I’d seen the document archive before, but was overwhelmed by the volume]. I’d failed to see the key that allows finding the various threads among the array of files [I really appreciate her sending it]. I’ve spent a few days perusing the memos and emails. It’s pretty incriminating stuff – people talking about how to hide or minimize negative studies, how to avoid mentioning side effects, having affairs with each other, how to hype off label uses, how to spin all sorts of things – incriminating and sometimes disgusting. As I’ve read through the evidence, I keep wondering how all this seedy business played out in getting the drug through the F.D.A. and on the market [and into such widespread and lucrative use].
Another great post, glad the documents were/are of interest! I am looking forward to your answer(s) to the question I have…same one..how did the drug get approved, and even moreso, why is it continuing to get wide spread approval use, such as in age 10 and up, etc. Was someone in the FDA paid off? so many questions!
Great to hear you are on the case.
many thanks
Mickey–
Great site . . . I visit daily. Any chance I can get an e-mail/snail mail for you. I would like to send you a copy of a letter I didn’t send to the Georgia Medical Association that shows psychiatrists are not the only physicians who are cavalier in their dispensation of atypicals.
Astra has earned its chops along the way:
http://www.businessweek.com/1996/28/b348355.htm
[…] A more cynical argument for why antipsychotic drugs don’t “separate from placebo” is because they really aren’t that much better than placebo (for an excellent series of posts deconstructing the trials that led to FDA approval of Seroquel, and showing how results may have been “spun” in Seroquel’s favor, check out 1BoringOldMan). […]
Thank-you “not so”boring old man for the info. quite interesting.