In another life long ago, I was an N.I.H. Fellow in "hard science" and as part of my Fellowship, they offered a chance to get a Master’s Degree in Statistics. I was young with lots of energy, so I took all the courses, but I never got it because you had to submit a paper, and I got whisked off to be a soldier, blah, blah, blah. The point was that I really liked it. We had some fine teachers. The group was all PhD.s in some kind of medical science and one Physician – me. We had a lot of fun, and since all of us were involved in research projects, we learned using "real" data. Once, our best professor said something like this. ‘In your careers, no matter what I say, you’re going to present your own data in the best light possible. That’s what people do. I can’t stop you. What I can say is don’t use statistics to try to make shit shine.’ At another time, he said, ‘Before you start prettying everything up, show your data in the rawest possible form. If you can see differences in the raw data, use your statistics to confirm your eyes. If you can’t see it in the data itself, even if your statistics find something, it’s usually trivial.’ So we brought in our raw data and he got us to guess what it would show before we headed for the computer [back in the days of "punch cards" and overnight main-frame runs] or the Frieden [rat-a-tat-tat] Calculators. I told you we had good teachers.

 I thought about those days when I looked at this next study [Trial 0012]. Put the graphs on your screen, then roll your chair back six feet. You’ll see why. This was a European study that was both presented and published.
I thought about those days when I looked at this next study [Trial 0012]. Put the graphs on your screen, then roll your chair back six feet. You’ll see why. This was a European study that was both presented and published.Seroquel (ICI204636): AnAtypical Antipsychotic- Results from Phase III
European Neuropsychopharmacology Volume 6, Supplement 3 , Page 202, June 1996.
by DJ. King 1, C.G.G. Link
Department of Therapeutics & Pharmacology
The Queen’s University of Belfast, Northern Ireland
Zeneca Pharmaceuticals, Medical Research Department
Alderley Park, Macclesfield, Cheshire, England‘Seroquel’ (lCI 204636) is a dibenzothiazepine atypical antipsychotic agent. In phase 2 trials, ICI 204636 has been shown to be an effective antipsychotic with a low EPS potential. The SAFARI study was a 6 week, multicentre, randomised, parallel group comparison of ICI 204636, 225 mg bd, 150 mg tid and 25 mg bd in hospitalised patients with acute exacerbation of schizophrenia (DSM I1IR).The primary objectives of the study were to compare the clinical efficacy of 450 mg per day dosed bd vs tid with reference to a low dose of 50 mg per day on positive and negative symptoms (CG!, BPRS and SANS scores) and tolerability (AIMS and Simpson score for EPS). Results were analysed by ANCOVA with last observation carried forward. A total of 618 patients entered the randomised portion of the trial. On day 42, relative to the 25 mg bd group, 225 bd was shown to improve mean CGI score (p :’S 0.05) and reduce BPRS total score (p :’S 0.05) and SANS summary score (p=0.02). The 225 mg bd and 150 mg tid group were not distinguishable on BPRS, SANS or CG!. Twice daily dosing of ICI 204636 (450 mg/day) had a similar tolerability profile to tid dosing but an improved EPS profile as shown by the Simpson score when patients were dosed bd (p =0.04). No regimen was associated with increases in prolactin. It was concluded that, although ICI 204636 had a relatively short plasma elimination t½, there were therapeutic advantages from dosing twice as opposed to three times daily.
‘Seroquel’ is a trademark the property of Zeneca Limited.
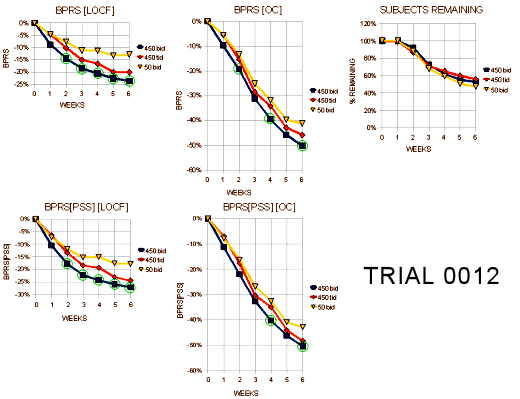

 I almost want to say that if you’re Schizophrenic, leave the U.S. I think their intention was for the 25mg bid [50mg.day] dose to be a homeopathic dose. But whether it was the very low dose or the non-American atmosphere, these patients improved a lot over the six weeks no matter what they were given to take. Here’s my graphic for the the "last observed" dose on the subjects who dropped out [a lot of late drop-outs here too]:
I almost want to say that if you’re Schizophrenic, leave the U.S. I think their intention was for the 25mg bid [50mg.day] dose to be a homeopathic dose. But whether it was the very low dose or the non-American atmosphere, these patients improved a lot over the six weeks no matter what they were given to take. Here’s my graphic for the the "last observed" dose on the subjects who dropped out [a lot of late drop-outs here too]: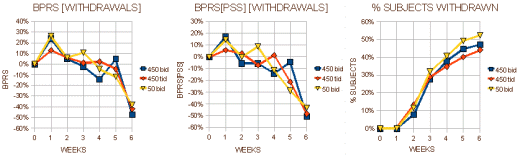
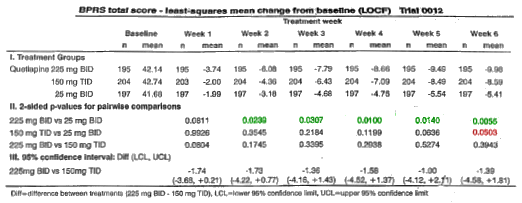
-
The 450mg dose [bid] is significantly different from the 50mg dose.
-
The 450mg dose [bid] is not significantly different from the 450mg [tid] dose.
-
The 450mg dose [tid] dose is [if anything] barely different from the 50mg dose.
My professor never said this, but I left his courses thinking it whether he said it or not. If there’s an obvious statistical operation that would answer some question, and the author didn’t do it, you’ve got to wonder if they did it but just didn’t like the way it came out.
Another F.D.A. report [Review and Evaluation of the Clinical Data] has a different version of the conclusion:
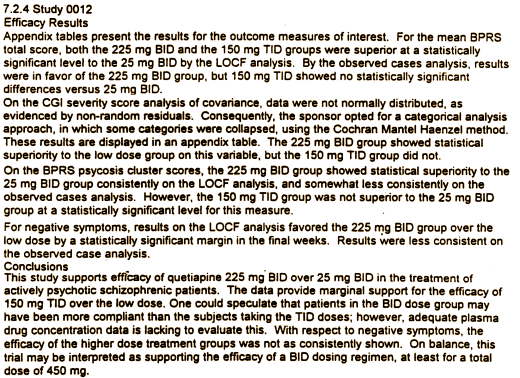
What a great quote: don’t use statistics to make your shit shine. What a shame the Borison’s, Nemeroff”s, Keller’s of the world haven’t heard it.
Please keep this up! You are doing a great job.
FYI
http://pharmagossip.blogspot.com/2011/02/what-were-astrazenecas-senior.html