We all know how the F.D.A. Approval comes out. Seroquel was approved in September of 1997 and turned into a megablockbuster for AstraZeneca and for the Patients [and their Lawyers] who sued [and will continue to sue] them. But let’s continue to look carefully at Trial 0013, the one that brought the approval home for then Zeneca Pharmaceuticals.

 I had known about Trial 0015 from reading those subpoenaed documents released in 2009, but I hadn’t known where it fit in the approval story. So when I first started looking at Trial 0013, because of the similarities in the protocols, I wondered if it wasn’t just a piece of Trial 0015 that they plucked out [the raw data is not available]. Were they pulling a fast one? That’s what sent me back going through the F.D.A. documents until I found it submitted with their approval package [see seroquel V: through the looking glass…]. It was a discrete study published in Biological Psychiatry:
I had known about Trial 0015 from reading those subpoenaed documents released in 2009, but I hadn’t known where it fit in the approval story. So when I first started looking at Trial 0013, because of the similarities in the protocols, I wondered if it wasn’t just a piece of Trial 0015 that they plucked out [the raw data is not available]. Were they pulling a fast one? That’s what sent me back going through the F.D.A. documents until I found it submitted with their approval package [see seroquel V: through the looking glass…]. It was a discrete study published in Biological Psychiatry:Multiple Fixed Doses of "Seroquel" (Quietiapine) in Patients with Acute Exacerbation of Schizophrenia: A Comparison with Haloperidol and Placebo
by Lisa A. Arvanitis, Barbara G. Miller, and the Seroquel Trial 13 Study Group
BIOL PSYCHIATRY 1997;42:233-246
Five fixed doses of the atypical antipsychotic "Seroquel" (quetiapine) were evaluated to delineate a dose-response relationship, as measured by changes from baseline in Brief Psychiatric Rating Scale (BPRS), Clinical Global Impression (CGI), and Modified Scale for the Assessment of Negative Symptoms (SANS) summary scores, and to compare efficacy and tolerability opposite placebo and haloperidol. Three hundred sixty-one patients from 26 North American centers entered this double-blind, placebo-controlled trial with acute exacerbation of chronic schizophrenia (DSM-III-R). Patients who completed a single-blind, placebo washout phase were randomized to double-blind treatment with quetiapine (75, 150, 300, 600, or 750 mg daily), haloperidol (12 mg daily), or placebo and evaluated weekly for 6 weeks. At end point, significant differences (p < 0.05, analysis of covariance) in adjusted mean changes from baseline were identified between the four highest doses of quetiapine and placebo for BPRS total, BPRS positive-symptom cluster, and CGI Severity of Illness item scores and between quetiapine 300 mg and placebo for SANS summary score. Differences between quetiapine and haloperidol were not significant. Dose-response modeling showed significant linear and quadratic functions of quetiapine dose for all primary efficacy variables. Notably, no significant safety concerns were identified as dose increased. Quetiapine was no different from placebo across the dose range studied regarding incidence of extrapyramidal symptoms or change in prolactin concentrations. Quetiapine is well tolerated and clinically effective in the treatment of schizophrenia. It is both superior to placebo and comparable to haloperidol in reducing positive symptoms at doses ranging from 150 to 750 mg/day and in reducing negative symptoms at a dose of 300 mg/day. [Received August 14, 1996; revised March 18, 1997.]
Here are the F.D.A. Report’s comments and some of the data:
"This Phase III trial randomized a total of 361 patients among 26 in the US and Canada to 5 fixed doses of Seroquel (75 mg N=53, 150 mg N=48, 300 mg N=52, 600 mg N=51, 750 mg N=54) or 12 mg haloperidol (N=52) or placebo (N=51). The target sample size of 50/arm resulted from simulations of various dose-response models using a power of 90%."
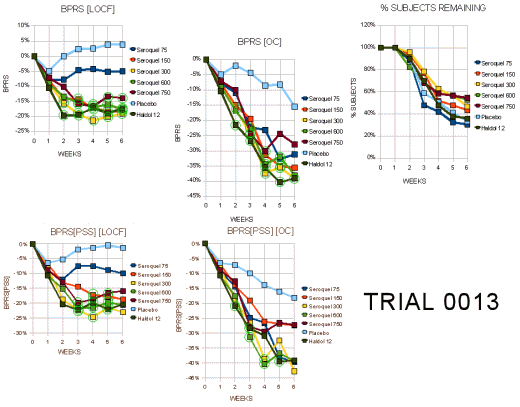
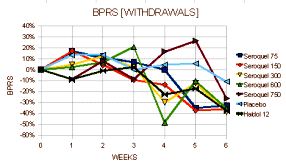

 Lots and lots of green circles on this study. But before we get to that, look at the Haldol response. Haldol appears to be the gold standard in this study, usually surpassing that of Seroquel at any dose. We knew that from Trial 0015, though that study wasn’t considered by the F.D.A. apparently. They chose a fairly brisk dose of Haldol which probably explains the fact that the Haldol had the largest drop-out rate of any group [my guess – side effects]. But we know from Trial 0015 that’s not the case long-term [seroquel V: through the looking glass…]. This study is actually an example of one of the things wrong with the LOCF method. In the placebo and low dose Seroquel groups, the drop-outs are likely a function of worsening untreated disease. In the Haldol group, the drop-outs are probably from side effects. Such push-pull effects make the results confusing [eg the "last observed" at withdrawal curves]. In the report, the reviewers said:
Lots and lots of green circles on this study. But before we get to that, look at the Haldol response. Haldol appears to be the gold standard in this study, usually surpassing that of Seroquel at any dose. We knew that from Trial 0015, though that study wasn’t considered by the F.D.A. apparently. They chose a fairly brisk dose of Haldol which probably explains the fact that the Haldol had the largest drop-out rate of any group [my guess – side effects]. But we know from Trial 0015 that’s not the case long-term [seroquel V: through the looking glass…]. This study is actually an example of one of the things wrong with the LOCF method. In the placebo and low dose Seroquel groups, the drop-outs are likely a function of worsening untreated disease. In the Haldol group, the drop-outs are probably from side effects. Such push-pull effects make the results confusing [eg the "last observed" at withdrawal curves]. In the report, the reviewers said:"Withdrawal rates ranged from 50%-70% with the highest in the placebo, 75 mg and haloperidol groups. Tables 16a and 16b display the visit-wise results of the total BPRS for the LOCF and observed cases analyses, respectively. All but the 75 mg/day dose of Seroquel were statistically significantly different from placebo for the LOCF analysis, whereas none were significant in the completers analysis… Tables 18a and 18b display the results of the key positive symptom BPRS cluster for the LOCF and observed cases, respectively. This endpoint shows a similar profile of a nearly significant LOCF analysis and a null completers analysis."
In spite of all the green circles [significant values], at the end, the BPRS was not significant in the OC [completers] graph, and in neither the LOCF or OC graph for the BPRS[PSS]. Here was their comment in the Review and Evaluation of the Clinical Data report:
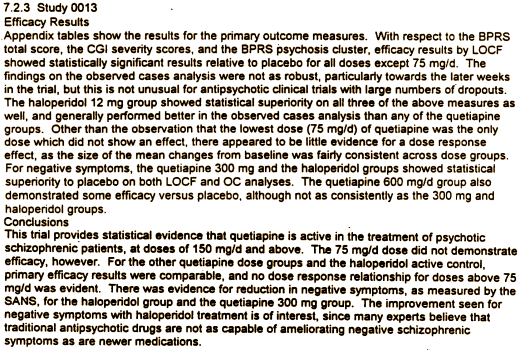
Notice the comment, "… and no dose response relationship for doses above 75mg was evident." Dr. Lisa Arvanitis thought otherwise in her published version, "Dose-response modeling showed significant linear and quadratic functions of quetiapine dose for all primary efficacy variables." For the moment, we’ll forgo her comments about weight gain. As for EPS, Seroquel wins hands down:
| Extrapyramidal Symptoms | |||||||
| Placebo | Seroquel 75 mg |
Seroquel 150 mg |
Seroquel 300 mg |
Seroquel 600 mg |
Seroquel 750 mg |
Haldol 12 mg |
|
| (n = 51) | (n = 53) | (n = 48) | (n = 52) | (n = 51) | (n = 54) | (n = 52) | |
| EPS (all) | 9(18) | 4 (8) | 3 (6) | 2 (4) | 4 (8) | 3 (6) | 19(37) |
| Akathisia | 4 (8) | 1 (2) | 1 (2) | 0 | 0 | 1 (2) | 8(15) |
| Parkinsonism | 5(10) | 3 (6) | 2 (4) | 2 (4) | 4 (8) | 2 (4) | 15(29) |
| Dystonia | 1 (2) | 1 (2) | 0 | 0 | 1 (2) | 0 | 1 (2) |
Her overall conclusion:
In conclusion, this trial, as well as earlier trials, has shown that Quetiapine is clinically effective in the treatment of acute exacerbation of chronic or subchronic schizophrenia. Superiority to placebo and comparability to haloperidol in reducing positive symptoms were shown across a dose range of 150-750 mg/day and in reducing negative symptoms at a dose of 300 mg/day. Determining Quetiapine’s effects on other domains of psychopathology [e.g., primary negative symptoms, cognitive function, quality of life] and in other patient populations, as well as comparing Quetiapine’s efficacy and tolerability with that of newer antipsychotics, are subjects for future study.
Before taking a breather to contemplate all of this information, let’s note a familiar addendum to each of Dr. Arvanitis’ studies that was also present in this one:
This trial was supported by a grant from Zeneca Pharmaceuticals. The authors thank Suzanne Bristow-Marcalus, BSP, ELS, for writing support, Jean Fennimore, BS RN, for trial organization and management support, Jacqueline Fiore and Gary Fritz for trial monitoring support, and members of the Seroquel data management team.
Sorry, the comment form is closed at this time.