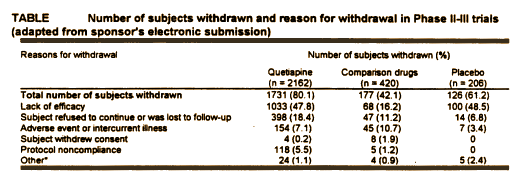
Zeneca submitted eight studies to the F.D.A. in their final approval request. Only four were considered for efficacy, but the other four were considered in the F.D.A.’s evaluation for safety. These studies involved years, a lot of money, multiple investigators and staff, and a large cohort of Schizophrenic patients [2788]. This Table is from the safety part of the the report [
Review and Evaluation of the Clinical Data] and includes all studies:
80% of the Seroquel treated subjects withdrew from their respective Clinical Trials before they were completed, the majority leaving for lack of efficacy [at about the same rate as in the Placebo group]. So we can rule out miracle cure or wonder drug status right off the bat.
One thing that’s clear from these studies, Schizophrenia isn’t well adapted to the world of Clinical Trials. Patients with an exacerbation of their illness are often in an escalating changing state, and prone to be suspicious of treatment [and everything else]. Given Placebos or ineffective doses of medication, their symptoms worsen and they bolt from the study. Likewise, they may flee because of side effects. Sometimes, they just flee under the best of circumstances for irrational reasons [it’s an irrational illness]. Any cohort of Schizophrenic patients is a heterogeneous group – some worsening, some improving, some refractory, etc. So the investigators are trying to hit a moving target and end up using techniques like LOCF, perhaps appropriate for occasional missing values, to deal with a majority of their study group disappearing prematurely.
In the case of
Zeneca‘s
Seroquel hegira, I’m looking at it in part because we think that the Pharmaceutical Company stepped over some lines in their quest to get the drug approved. In the four published reports, there’s no question of ghost-writing or conflict of interest, because that’s all there was. All studies were funded by
Zeneca. One, two, or three of the authors were on the
Zeneca staff [Arvanitis LA, Miller BG, Link CCG]. And I very much doubt that Dr. Richard Borison, first author on the
Trial 0006 was personally involved in the writing as he was
busy buying suits of armor for his castle at the time. I don’t know about Drs. Small, Hirsh, or King, but apparently figurehead authorship was fashionable in the late 1990s. The three full articles were either written by or at least heavily edited by a contract medical writer and a professional data management staff provided by the company was involved.
This study was supported by a grant from Zeneca Pharmaceuticals, Macclesfield, England, and Wilmington, Del. We thank Suzanne Bristow-Marcalus for medical writing support; Jean Fennimore, RN, and Alison Smith, MSc, for trial organization and management support; Gary Cooper, MS, Jacqueline Fiore, Lesley Farrow, and Barney Home for trial monitoring support; and Kathleen Jelliffe, Denise Redkar-Brown, Joy Russo, and Thais Womack for data management support.
So these articles were definitely corporate productions extraordinaire, published in scientific journals [also fashionable in the late 1990s and later].
Did Zeneca cheat [right now we’re looking only at efficacy – safety comes later]? They certainly showed their best case graphs, tables, minimized the downsides, and overstated their conclusions in the published reports. In terms of their communications with the medical community, they kept the negative studies to themselves. They implied they had a powerhouse drug, when it was no better than the first antipsychotic ever discovered, Chlorpromazine, in Trial 0007; apparently lost out to Haldol in Trial 0014; and was decimated long-term by Haldol again in the withheld "cursed study," Trial 0015.
Rather than pick at the published reports, I’m going directly to the F.D.A. process. I was impressed with the F.D.A. reviews. They had the raw data and pored over it appropriately. Reading the report, I felt I was seeing the Seroquel that exists, not the one in Zeneca‘s fantasies. There were a couple of places where they drank the Kool-ade, noted previously. At the risk of mindless repetition, here’s a sampling of the relevant data:
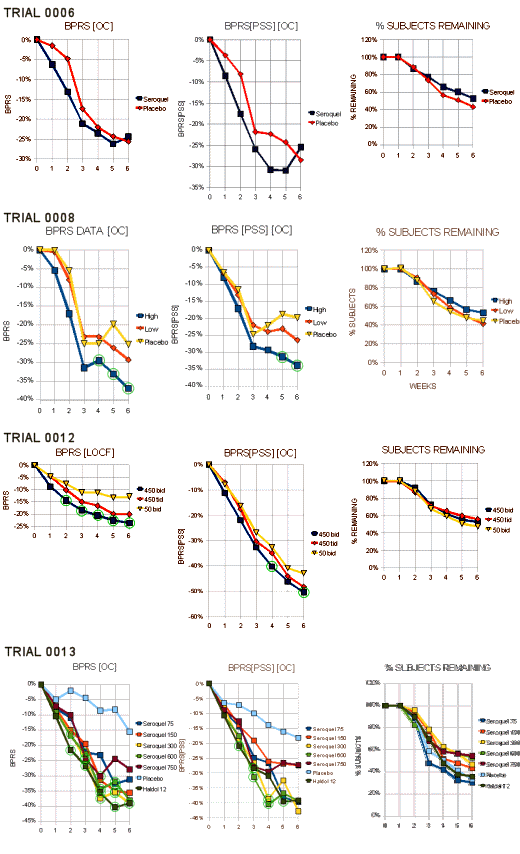
…The F.D.A. reviewer gave Zeneca Trial 0008 and Trial 0013. They only needed two…
"Trials 0008 and 0013 clearly demonstrate statistically signicantly differences between Seroquel and placebo. Both trials were able to demonstrate treatment differences of at least 4 total BPRS points in completers at six weeks. The reasons for the null results for completers in trial 0006 are unclear. The median doses of the Seroquel completers at the end of the trials were simar in both trials 0006 and 0008, another dose-escalation trial (450 rng and 500mg, respectively). The major difference was in the pedormance ofthe placebo groups. The placebo group in trial 0006 decreased from baseline an average of 13.9 points while the placebo average in trial 0008 was 9.7, making it more difficult to show a difference in trial 0006. As a checkon trial 0008, this reviewer analysed ony the first 165 randomized patients and foundthe treatment difference to be comparable to that using all patients. In a submission dated September 24, 1996, the sponsor reanalyzed the data without Dr. Borison’s center. Omitting Dr. Borison’s center had no substantial effect on the results."
What they didn’t do was consider
Trial 0015. I don’t know if the F.D.A. wasn’t allowed to consider it; if they bought Lisa Arvanitis’ "smoke and mirrors" job in the write-up; or if they believed their own peculiar comment, "
… and failed to show a difference in relapse rates among treatment groups. Thus it did not generate meaningful efficacy data" [
seroquel V: through the looking glass…]. I know I’m a biased observer. I already know what’s coming – the internal documents, the off-label campaigns, the approval creep, the speaker’s bureaus, the deceitful side-effect cover-up, and so on. But I’m also biased because I’m a clinician who has seen this drug used to treat the very disease we’re talking about, Schizophrenia, and I’ve been way disappointed. That said, I think my reaction to this data would be the same without my bias [who ever knows?]. Without
Trial 0015, I would say, "Okay. You win. It
is an Antipsychotic, but a real lightweight – a non-robust treatment for this robust illness. You shouldn’t have to go to this much trouble just to prove that it works. I’ll bet you’re disappointed too."
But then… here is Trial 0015 again:
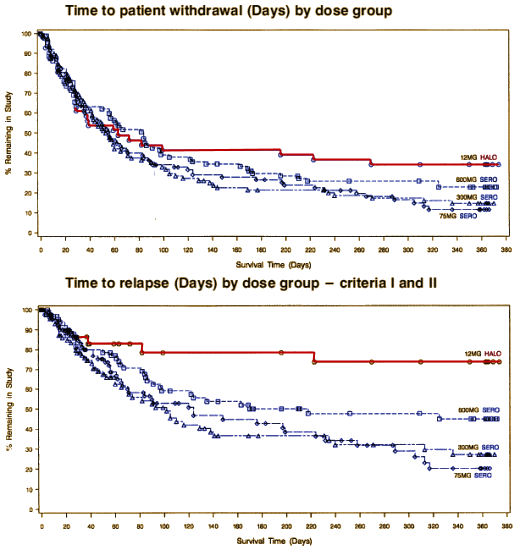
With that in front of me, Zeneca is going to have to have some very impressive side effect data. The whole point is relapse prevention without long term toxicity, and this relapse prevention profile is mighty lame. So, yes, they did cheat – cheating by ommission. They did a study that really mattered, and they didn’t put it on the table for the F.D.A. directly, nor at all for those of us involved with advising people with a major disorder about its use. I don’t know what effect that would’ve had on getting it approved, but it would’ve allowed clinicians and patients a clear view of what to expect. And this statement at the end of Dr. Arvanitis’ paper may be true in the particulars, but implies something that is a not true – and she knew it [an overall comparability to haloperidol]. Reading her paper, I couldn’t have known that Seroquel is not a very good maintenance medication:
In conclusion, this trial, as well as earlier trials, has shown that Quetiapine is clinically effective in the treatment of acute exacerbation of chronic or subchronic schizophrenia. Superiority to placebo and comparability to haloperidol in reducing positive symptoms were shown across a dose range of 150-750 mg/day and in reducing negative symptoms at a dose of 300 mg/day.
And as for evidence-based medicine and the Clinical Trial world – there’s only one graph in this whole series that is self evident [Trial 0015]. All the others have to be explained and obsessed about. I find little here that is any clearer than the subjective world of my own career as a psychotherapist. So now on to the question of corporate morality, side effects, and safety…


You’ve probably seen this, but just in case
http://i.bnet.com/blogs/spielmans-parry-ebm-to-mbm-jbioethicinqu-2010.pdf
From Evidence-based Medicine to Marketing-based
Medicine: Evidence from Internal Industry Documents
Glen I. Spielmans & Peter I. Parry
I bow at your feet. You are doing such a great job
Thank you – you don’t know how much this means…..
Maybe one day I’ll be able to explain!