1. at least as efficacious as other first line atypicals
2. more effective than haloperdol and chlorpromazine
3. effective at preventing depression and improving cognition
4. superior tolerability to typicals and first line atypicals
5. weight neutral, placebo-level EPS and prolactin levels, and no significant QT prolongation
That study’s primary outcome measure was simple:
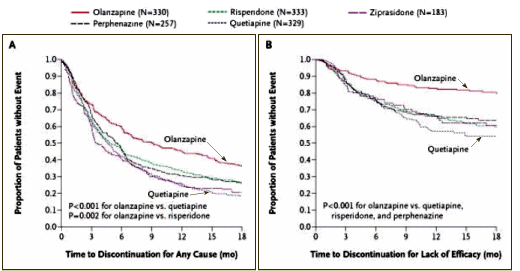
We hypothesized that there would be significant differences in the overall effectiveness of olanzapine, perphenazine, quetiapine, risperidone, and ziprasidone in treating schizophrenia that reflected variations in efficacy and tolerability. The primary outcome measure was the discontinuation of treatment for any cause, a discrete outcome selected because stopping or changing medication is a frequent occurrence and major problem in the treatment of schizophrenia. In addition, this measure integrates patients’ and clinicians’ judgments of efficacy, safety, and tolerability into a global measure of effectiveness that reflects their evaluation of therapeutic benefits in relation to undesirable effects.
Seroquel [Quetiapine] didn’t fare so well:
And so another couple bit the dust:
1. at least as efficacious as other first line atypicals
4. superior tolerability to typicals and first line atypicals
CAFE found equal efficacy for Olanzapine [
Zyprexa], Quetiapine [
Seroquel], and Risperidone [
Risperidol]. That study has been both criticized and defended [I’ll pass on joining in that debate – see
Carlat‘s discussion and the first two comments on his post, and
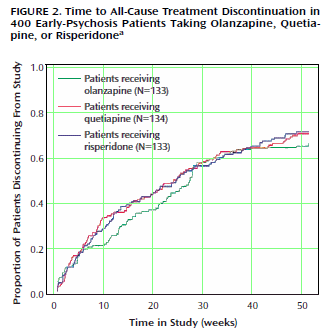
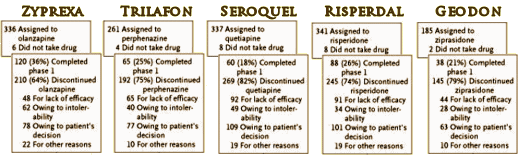
The Seroquel Scandal: A Minnesota Psychiatrist’s Ethical Lapses Are Suspected]. To me, the remarkable numbers in both studies are the overall drop-out rates:
| |
OVERALL DROP-OUT RATE |
| |
Zyprexa |
Trilafon |
Seroquel |
Risperdal |
Geodon |
| |
|
| CATIE[18 months] |
64% |
75% |
82% |
74% |
79% |
| CAFE [12 months] |
68% |
· |
71% |
71% |
· |
So I’ll partially restore one, giving AstraZeneca the benefit of the doubt for the moment:
1. at least sometimes as efficacious as other first line atypicals
There goes that benefit of the doubt:
1. at least as efficacious as other first line atypicals
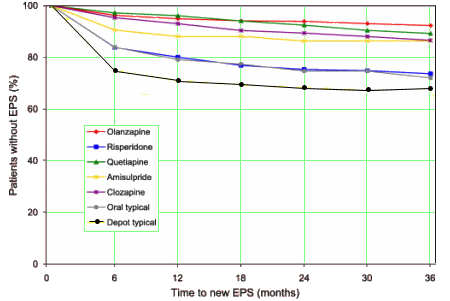
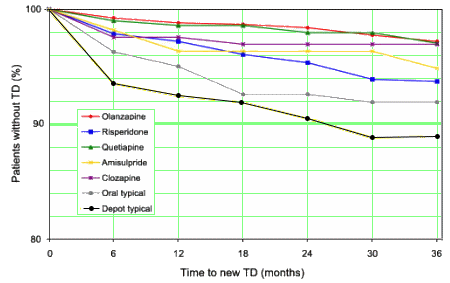
And as for Extrapyramidal Symptoms and Tardive Dyskinesia, that study compared the incidence among the Atypicals and also included the Typicals:
5. weight neutral, placebo-level EPS and prolacin levels, and no significant QT prolongation
Although the incidence of EPS and TD is low with
Seroquel, it is not "placebo-like." So, in the decade since the
AstraZeneca Seroquel Strategic Plan of 2000 listed their five
Key Claims:
1. at least as efficacious as other first line atypicals
2. more effective than haloperdol and chlorpromazine
3. effective at preventing depression and improving cognition
4. superior tolerability to typicals and first line atypicals
5. weight neutral, placebo-level EPS and prolactin levels, and no significant QT prolongation
and #3. is without evidence as well.
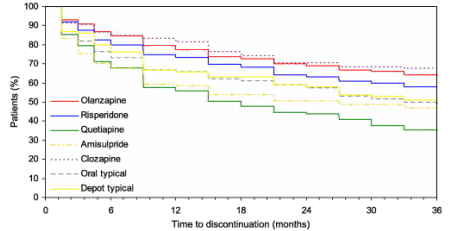
While I’m focusing on Seroquel and the behavior of AstraZeneca in marketing the drug, they weren’t alone. Eli Lilly was fined $1.4 B for off-label promotion of Zyprexa. But there’s another point in all of this. Most Psychiatrists were as hopeful as their manufacturers that the Atypical Antipsychotics would be effective antipsychotics without the downside of the first generation drugs. They aren’t. While the EPS/TD incidence is lower, it remains a problem. The weight gain and "metabolic syndrome" with possible Diabetes is ominous. And the patients themselves discontinue these medications at least as often as the older drugs, and sometimes more frequently. So the drug companies, the schizophrenic patients, their families, and their Psychiatrists are disappointed. We all had high hopes, and the story didn’t play out like any of us wanted.
The problem isn’t Seroquel. It just is what it is. The intrusion of the pharmaceutical industry in the process of our knowing [or not knowing] about the drug – the things they should’ve told us along the way – is a problem. Worse, they actively lied about the drug for years. But the biggest problem is that a medical profession allowed it all to happen, more than a few even colluding in the deception…
Thanks Mickey.
Clearly there were some amoral people directing this inside the company.
However, how did so many people in a big organization like AZ allow it to happen?
Many employees (and others) were complicit.
“Complicity works like this. All those with a vested interest in an enterprise get sucked into the rhetoric associated with it, and they soon `believe’ in everything that is going on within that enterprise. If personal financial gain is involved, corruption may also occur.” – Paul Dieppe
There is a literature on “Complicity Theory”. This might help explain what happened.
And as you say: What of the psychiatrists and “KOLs” – where was their objectivity?
You can add coronary heart disease to the list (although the authors conflate patients being on antipsychotics with patients with psychotic symptoms. In the methods, they refer to patients being treated, and this is the basis for my interpretation of the results):
Schizophr Res. 2011 Feb;125(2-3):295-9. Epub 2010 Nov 19.
Increased Framingham 10-year risk of coronary heart disease in middle-aged and older patients with psychotic symptoms.
Jin H, Folsom D, Sasaki A, Mudaliar S, Henry R, Torres M, Golshan S, Glorioso DK, Jeste D.
Department of Psychiatry, University of California, San Diego, CA 92161, United States.
OBJECTIVE: The Framingham 10-risk of coronary heart disease (CHD) has been a widely studied estimate of cardiovascular risk in the general population. However, few studies have compared the relative risk of developing CHD in antipsychotic-treated patients with different psychiatric disorders, especially in older patients with psychotic symptoms. In this study, we compared the 10-year risk of developing CHD among middle-aged and older patients with psychotic symptoms to that in the general population.
METHOD: We analyzed baseline data from a study examining metabolic and cardiovascular effects of atypical antipsychotics in patients over age 40 with psychotic symptoms. After excluding patients with prior history of CHD and stroke, 179 subjects were included in this study. Among them, 68 had a diagnosis of schizophrenia, 42 mood disorder, 38 dementia, and 31 PTSD. Clinical evaluations included medical and pharmacologic treatment history, physical examination, and clinical labs for metabolic profiles. Using the Framingham 10-year risk of developing CHD based on the Framingham Heart Study (FHS), we calculated the risk CHD risk for each patient, and then compared relative risk in each psychiatric diagnosis to the risks reported in the FHS.
RESULTS: The mean age of entire sample was 63 (range 40-94) years, 68% were men. The Framingham 10-year risk of CHD was increased by 79% in schizophrenia, 72% in PTSD, 61% in mood disorder with psychosis, and 11% in dementia relative to the risk in general population from the FHS.
CONCLUSIONS: In this sample of middle-aged and older patients with psychotic symptoms, we found a significantly increased 10-year risk of CHD relative to the estimated risk from FHS, with the greatest increased risk for patients with schizophrenia and PTSD. (emphases mine)
“But the biggest problem is that a medical profession allowed it all to happen”
Hear, hear.
Far be it from me to defend the pharmaceutical industry, but many of their tactics aren’t quite as hidden as those you have so expertly uncovered, and should have been observed and commented upon by our profession long before now. Heck, even today I find it shocking how easily some of my colleagues accept the marketing messages being handed to them by sales reps, in glossy brochures, and in promotional throwaway journals, all of which have little to do with the reality of the patients in their offices.
It’s time for us to act like the intelligent, educated adults we are, and make decisions accordingly.
Thanks again for this excellent series.