| There must be two sides to every story, And whose to say whose right and who is wrong… |
| Willie Nelson – Honeysuckle Rose |
In prehistory, Pharmaceutical Clinical Trials were arranged by the Drug Companies directly – often by contract with academic institutions that weren’t in the running for the more competitive N.I.M.H. research funding. In the last three decades, there’s a new landscape in clinical research. Since I grew up in the Medical world of the 1960s, 1970s, and early 1980s, then entered practice in a specialized area distant from the domain of pharmacology, I might as well have landed in Munchkin-Land when I started trying to explore the modern universe of clinical research – a stranger in a strange land. Apparently, one of the forces that created the modern Clinical Research Industry was speed. Recruiting patients for drug studies was not at the top of the list for academic institutions, so things moved slowly waiting for appropriate patients for a given study to come along.
Clinical Research Centers
What has evolved is a vast global network of Clinical Research Centers in academic institutions, private hospitals, and "centers" in a diverse array of settings. Just put Clinical Research Center into Google and you’ll be greeted with a gajillion hits and a map of the ones near you. Click on a center and you’ll get a list of the their current Clinical Trials for you to join. Since the key to running a successful Clinical Research Center is recruitment, they’re located wherever potential subjects might congregate – all over the world.
 Clinical Trials Research has become a profession. You can even get a Masters Degree in the field. There’s a professional organization [ACRP – Association of Clinical Research Professionals] offering approved certification [now optional] and an organization for Physicians who are involved with these Centers [APPI – Academy of Pharmaceutical Physicians and Investigators]. The rise of the Clinical Research Center
Clinical Trials Research has become a profession. You can even get a Masters Degree in the field. There’s a professional organization [ACRP – Association of Clinical Research Professionals] offering approved certification [now optional] and an organization for Physicians who are involved with these Centers [APPI – Academy of Pharmaceutical Physicians and Investigators]. The rise of the Clinical Research Center 
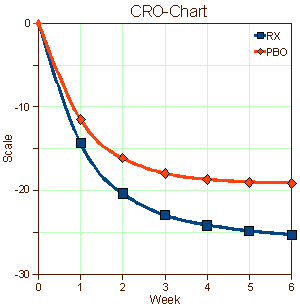
 Industry has accelerated the time-to-market for new drugs but there’s been a price. Since the premium is on rapid recruitment often by direct advertisement, the subject populations are more diverse and less ill than formerly. This is one of the forces that accounts for the "placebo droop" in many such trials [the large response to placebos in the CRO-Charts].
Industry has accelerated the time-to-market for new drugs but there’s been a price. Since the premium is on rapid recruitment often by direct advertisement, the subject populations are more diverse and less ill than formerly. This is one of the forces that accounts for the "placebo droop" in many such trials [the large response to placebos in the CRO-Charts].  With a healthier subject base, larger numbers of subjects are required to achieve the kind of significance required by the F.D.A. Like Wille Nelson says, there must be two sides to every story. Recently, Vanity Fair published a scathing picture of the Clinical Research Centers [Deadly Medicine]. The ACRP has an response to the article [ACRP Responds to Vanity Fair’s "Deadly Medicine"]. For the moment, I’ll stay out of whose right and who is wrong.
With a healthier subject base, larger numbers of subjects are required to achieve the kind of significance required by the F.D.A. Like Wille Nelson says, there must be two sides to every story. Recently, Vanity Fair published a scathing picture of the Clinical Research Centers [Deadly Medicine]. The ACRP has an response to the article [ACRP Responds to Vanity Fair’s "Deadly Medicine"]. For the moment, I’ll stay out of whose right and who is wrong.
Clinical Research Organizations
If you read much of the medical literature, you’ve probably noticed that the articles often say something like "at 37 centers" and may even list the sites at the end of the paper. I guess I thought that the Drug Company farmed the trials out themselves, but that’s not the usual case. And when you read something like "writing support by William Faulkner of Yoknapatawpha" in the acknowledgments, it doesn’t occur to the uninitiated that these are references to a central player in this game – the Clinical Research Organizations [CROs]. In fact, many of the functions we think of as performed by the drug companies are outsourced – and CROs are the "out" where they go these days.
I expect that the rise of the Clinical Research Organizations was inevitable. Pharmaceutical Companies [referred to as "Sponsors"] have a number of drugs on the market [under patent] followed by new drugs in development [in the "pipeline"]. Once they have developed a medication, they’re a long way from being able to sell the drug [called "time-to-market"] and there are lots of trials [called "phases"] along the way. Since they only have a few drugs in their pipeline, maintaining the support staff to jump through all the hoops is a big expense. The CROs [often started by former Pharmaceutical employees] got their start as a more cost effective way for the drug companies to go through these processes. So it’s hard to define what CROs do because the answer is a lot of things the drug companies used to do plus a bunch of new things the CROs thought up along the way. Unlike the CRCs that range from cottage industries to large University Centers, the CROs tend to be big corporations with lots of staff. While there are about 1000 CROs world-wide, over half the business is done by the top ten companies:
 |
|
| Quintiles [$2.5B, 15% marketshare 2009] | Icon [$887M] |
| Pharmaceutical Product Development [$1.4B] | Kendle [$590M] |
| Covance [$1.8B] | PharmaNet Development Group [$470M] |
| Charles River Laboratories [$1.2B] | PRA International [$410M] |
| Parexel [$930M] | INC Research |
If you’re interested in how the Pharmaceutical Industry works, it’s worth your time to peruse some of the CRO web-sites yourself. Be warned – these people speak in M.B.A. business-speak and if, like me, you’re not a native speaker, it may require rereading. An easy example to test your literacy [Like that line in Amadeus, "Too many words"]:
 The CROs are more than consultants. They offer direct services throughout the "life-cycle" of the drug: designing the trials; organizing the Physician Advisory Boards; contracting with the CRCs; shepherding the trials; collecting the results; analyzing results; dealing with the regulatory agencies; writing reports etc; writing articles [?]; planning and organizing launch campaigns; recruiting [?], managing, and supporting KOLs; post-marketing drug trials; advertising campaigns [?]; risk management; etc. The Sponsors "outsource" as many of these functions as they are willing to pay for, and the drug company and CRO staff mix and mingle on the various projects. As best I can tell, the CROs have several products: minimizing the Pharmaceutical’s operating cost, speeding the drug to market, and maximizing the Pharmaceutical’s profits [including risk management]. They have certainly expanded from simply being involved in Clinical Research.
The CROs are more than consultants. They offer direct services throughout the "life-cycle" of the drug: designing the trials; organizing the Physician Advisory Boards; contracting with the CRCs; shepherding the trials; collecting the results; analyzing results; dealing with the regulatory agencies; writing reports etc; writing articles [?]; planning and organizing launch campaigns; recruiting [?], managing, and supporting KOLs; post-marketing drug trials; advertising campaigns [?]; risk management; etc. The Sponsors "outsource" as many of these functions as they are willing to pay for, and the drug company and CRO staff mix and mingle on the various projects. As best I can tell, the CROs have several products: minimizing the Pharmaceutical’s operating cost, speeding the drug to market, and maximizing the Pharmaceutical’s profits [including risk management]. They have certainly expanded from simply being involved in Clinical Research. Medical Writing
 While most CROs advertise medical writing and medical communications, there are also a lot of medical writers who either freelance or work for medical writing companies. Such people often offer other services – meeting planning, presentation planning, etc, but don’t cover all the bases like the CROs An example would be Scientific Therapeutics Information, famous for Sally Laden and the ghostwriting instances recently unearthed by POGO. Medical writers also have educational programs, certification, and a professional organization, AMWA – American Medical Writers Association.
While most CROs advertise medical writing and medical communications, there are also a lot of medical writers who either freelance or work for medical writing companies. Such people often offer other services – meeting planning, presentation planning, etc, but don’t cover all the bases like the CROs An example would be Scientific Therapeutics Information, famous for Sally Laden and the ghostwriting instances recently unearthed by POGO. Medical writers also have educational programs, certification, and a professional organization, AMWA – American Medical Writers Association.
While it’s tempting to start at the top as I did when looking at Seroquel – reviewing the published studies and the marketing strategies used to move the drug to blockbuster status, when it is a lightweight [and dangerous] drug – I think the right place to start is at the bottom with the actual Clinical Trials themselves. There’s something very wrong with these graphs used to get F.D.A. approval that transcends all the smoke and mirrors they use to pretty them up. The people behind these graphs [CRO-Charts] just aren’t the right kind of "sick." They get better just by being in a study. It seems to me that there has to be something very wrong at the level of the Clinical Research Centers. Those graphs don’t even approximate the patients we see in our practices…
Sorry, the comment form is closed at this time.