ACRO’s global advocacy program is focused around a set of core principles:
•Secure a consistent, stable regulatory environment tosupport the continued globalization of clinical research•Protect the safety of research participants globally• Expand participation in clinical trials by physician investigators and volunteers;special emphasis on minority and special populations• Ensure ease of access to datanecessary to conduct research while considering patient privacy•Promote industry participation in comparative effectiveness research• Encourage health information technology policies that include consideration of medical research needs•Advance policies that encourage innovation in pharmaceutical, biologic and regenerative medicine development• Support a strong FDA with expanded resources in the areasof regulatory science andinternational operations•Support the EMA and European Commission in their efforts to advance GCP compliance andenhance international inspection regimes• Oppose conflict of interest policiesthat unduly restrict research and innovation•Further efforts topromote translational research through NIH and other government-funded bodies
Executive Summary:
As clinical trials have become increasingly globalized over the past ten to fifteen years, the possibility of conducting studies that offer adequate subject protection and yield reliable results in emerging countries has understandably attracted considerable attention. In this analysis, we examine the facts regarding the current state of clinical research and the role that biopharmaceutical companies and their clinical research organization (CRO) partners play in ensuring that the dual goals of trial safety and quality are met. Although concerns have been raised about the globalization of biomedical research, the reality is that emerging countries play a vital role in the advancement of medical science. Clinical trials in these countries, particularly those with industry sponsorship, are conducted at the high standards necessary to obtain regulatory approval in major markets. In addition, the investments made by trial sponsors, which are frequently implemented by CROs, are a major contributor to improving the health systems and economies of the developing world…[long list of the wonders of doing international drug trials]Although legitimate concerns have been raised in the past about clinical trials in emerging countries, the ability to conduct high quality studies in these locations has been enormously improved over the previous ten to fifteen years. Rather than placing further barriers to drug development, efforts should be focused on enhancing the progress that has already been made while continuing to train and monitor researchers throughout the world to ensure their compliance with the highest standards. As this report demonstrates, much of this is already being done as part of the normal business practices of biopharmaceutical companies and CROs, all of whom have a major stake in a strong and improving clinical research environment.
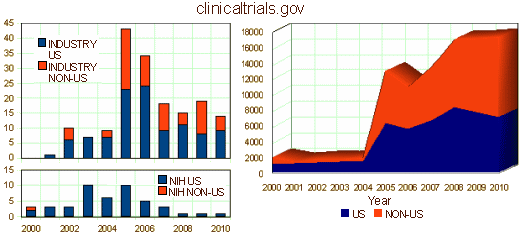
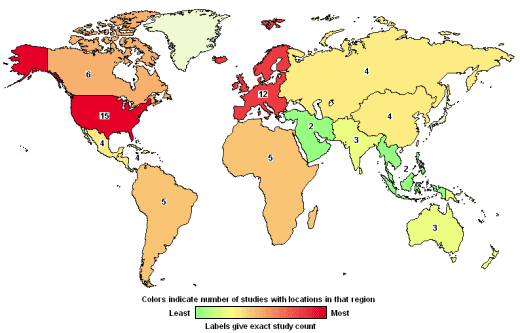
Along with globalization [which I’m sure makes recruitment easier and is less costly], they advocate for things that would make their jobs easier – data access and access to health information systems, international inspections [FDA, EMA, and European Commission], NIH translational research projects [moving bench science into health-care products and more Clinical Trials], and they oppose "restrictive" conflict of interest policies. Certainly, there is no reason to put up un-necessary blockades to their businesses. On the other hand, it’s clear that their business interests are the foremost concern of the CROs. As we will see when when look at the individual CROs, these business interests can easily conflict with the whole point of clinical trials, introducing a major bias to the enterprise. They advertise a speedy, cost efficient path to FDA approval. So they have a desired outcome – approval – and they’re in a hurry to get there. But the purpose of that approval process is to insure safety and efficacy, not the interests of the CROs. And, the liability for failure falls on the patients who take the drugs, the FDA that approves them, and the Pharmaceutical Manufacturers that sell them – not the CRO. There have been too many instances of premature or wrong approvals in recent years to respond to their pressures. We blame that on PHARMA, but CROs are part of the problem.
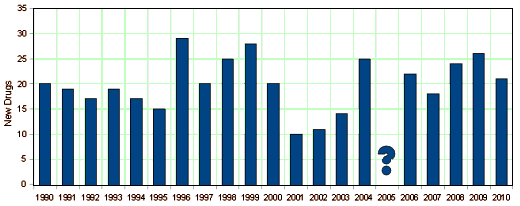
Additionally, there is an implied injunction to develop and approve more new drugs. While most of us might agree that it would be nice to have more safe and effective drugs, just having more isn’t desirable – particularly if they haven’t been carefully evaluated. It just makes the PDR thicker [and more dangerous]. I’ve stuck this post about their stated policies in before looking at the specific companies, because I think it’s important to note that those policies are self-serving, and may at times conflict with the actual goals of research altogether. Medicine separated itself from the ancient healing arts by one clear injunction – "Do no harm." It’s the counter to "therapeutic zeal." Proven safety and efficacy trump the natural wish to stomp out disease. If that’s not true, it simply isn’t medicine.
… it’s clear that their business interests are the foremost concern of the CROs… these business interests can easily conflict with the whole point of clinical trials, introducing a major bias to the enterprise. They advertise a speedy, cost efficient path to FDA approval. So they have a desired outcome – approval – and they’re in a hurry to get there.
Right. The CROs don’t talk much about clinical trials as tests of a hypothesis, and they don’t like to say that failure is an option. The CRO is the accoucheur of the experimercial. And when a trial conducted by a CRO does fail, is it ever acknowledged that the CRO failed in its job of quality control? Or does the candidate drug take the hit?
From Texas Attorney General Greg Abbott’s website: “Today’s multistate agreement stems from a complaint in federal court charging AstraZeneca with unlawfully marketing Seroquel for unapproved – or off-label – uses. The states also charged AstraZeneca with failing to disclose Seroquel’s harmful side effects and concealing scientific data that revealed safety concerns….” Read more here:
http://tinyurl.com/4fx82no