
After my recent Seroquel obsession, I thought it would be prudent to let it alone for a while, sort of percolate in my mind and see what bubbled up. I’ve spent a lot of time trying to figure out this business of Patents and Exclusivity [patent medicines…] thus far to no avail. So I was trying to leave Seroquel alone. But it won’t seem to leave me alone. Everywhere I turn, I see these ads. I can’t see the campaign as anything but a way for AstraZeneca to keep their cash cow working for them. And it’s hard for me to imagine that any rational clinician would prescribe a costly XR pill instead of the coming generics. But AstraZeneca must disagree, because those ads are everywhere. So I thought I’d look into the story of Seroquel XR to pass the time until I finally understand the Patent/Exclusivity story.
Seroquel XR: Schizophrenia…
| SEROQUEL | SEROQUEL XR | |
|
|
||
| Patent Number: | 4879288 | 5948437 |
| Patent Filed: | 03/20/1987 | 05/28/1997 |
| Patent Approved: | 11/07/1989 | 09/07/1999 |
| FDA Application: | N022047 | N022047 |
| FDA Approval: | 09/26/1997 | 05/17/2007 |
| Expiration: | 09/26/2011 | 03/28/2017 |
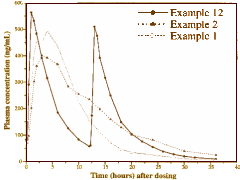 The Patent for Seroquel XR was issued in 1999 to Zeneca [Reading through it, I reached one immediate conclusion – not to reincarnated as a patent attorney]. What was patented was the process to make Quetiapine a sustained release medication. The figure shows the difference between bid doses and the extended release. The justification for why this is considered an "invention" and worthy of patenting isn’t clear from the document [but then again, the document isn’t particularly clear about anything else either]. Seroquel XR was approved by the FDA on 05/17/2007 for Schizophrenia ["maintenance treatment of schizophrenia"].
The Patent for Seroquel XR was issued in 1999 to Zeneca [Reading through it, I reached one immediate conclusion – not to reincarnated as a patent attorney]. What was patented was the process to make Quetiapine a sustained release medication. The figure shows the difference between bid doses and the extended release. The justification for why this is considered an "invention" and worthy of patenting isn’t clear from the document [but then again, the document isn’t particularly clear about anything else either]. Seroquel XR was approved by the FDA on 05/17/2007 for Schizophrenia ["maintenance treatment of schizophrenia"].
Psychiatry [Edgmont]. 2007 November; 4(11): 34–50.
by Joseph Peuskens, Jitendra Trivedi, Sergiy Malyarov, Martin Brecher, Ola Svensson, Frank Miller, Inger Persson, and Didier Meulien, on behalf of the Study D1444C00004 investigators
FUNDING: This study (no. D1444C00004) was supported by AstraZeneca Pharmaceuticals.DISCLOSURES: Dr. Peuskens has received research grants, consultancy, and lecture fees from AstraZeneca. Drs. Miller, Svensson, Brecher, Persson, and Meulien are employees of AstraZeneca; Dr. Svensson is a shareholder of AstraZeneca. Drs. Trivedi and Malyarov have no relevant conflicts of interest to disclose.ACKNOWLEDGEMENTS: …We also thank Dr. Catherine Hoare, from Complete Medical Communications Limited, who provided medical writing support funded by AstraZeneca.
AbstractIntroduction: This long-term, randomized, double-blind, placebo-controlled study examined the efficacy of extended release quetiapine fumarate [quetiapine XR] in preventing psychotic relapse in schizophrenia.Methods: Three hundred twenty-seven clinically stable patients with schizophrenia were switched to open-label quetiapine XR (300mg on Day 1, 600mg on Day 2, followed by flexible dosing [400–800mg/day]) for a 16-week stabilization phase. Thereafter, patients who were clinically stable for four months were randomized to flexible doses of quetiapine XR (400–800mg/day) or placebo. Primary endpoint was time to first schizophrenia relapse after randomization. Secondary endpoints included risk of relapse at six months. Interim analyses were planned after 45 and 60 relapses and final analysis after 90 relapses. Maximal treatment time was one year.Results: The study was terminated after the first interim analysis showed a significant difference between randomized treatment groups. Time to relapse was significantly longer in quetiapine XR-treated patients versus placebo (hazard ratio 0.16 [95% confidence interval 0.08, 0.34]; p=0.001). Fewer quetiapine XR-treated patients relapsed versus those receiving placebo (10.7% vs. 41.4%, respectively). Estimated risk of relapse at six months was significantly lower with quetiapine XR (14.3%) compared with placebo (68.2%; p=0.0001). The incidence of treatment-related adverse events (AEs) was similar between quetiapine XR and placebo groups (18% and 21% of patients, respectively) and only one percent of patients in each group withdrew because of AEs.Conclusion: Once-daily quetiapine XR (400–800mg/day) was effective in preventing relapse in patients with clinically stable schizophrenia. Quetiapine XR was well tolerated during longer-term use.
It was a modern Clinical Trial [Globalization] done at CRCs – 28 investigators at 26 sites [2 in Bulgaria, 4 in Poland, 9 in Russia, 6 in Ukraine and 5 in India]. This study was stopped early because it achieved the desired significance well before completion, making the results hard to evaluate since at the time the study was terminated, the subjects had been in it for a variety of time intervals. Here’s how the study went. 327 stable medicated Schizophrenic patients were recruited. As they entered the study, they entered a "stabilization phase" of 16 weeks – meaning they were changed from their current medication to Seroquel XR over four days. Of these subjects 67 were in this phase at the time the study was terminated [327-67=260]. Of that 260, 35 were not willing to continue and 28 were dropped for other reasons [260-35-28=197]. So, 197 subjects were randomized between Seroquel XR and Placebo. After 45 relapses, they’d achieved significance and terminated the study.
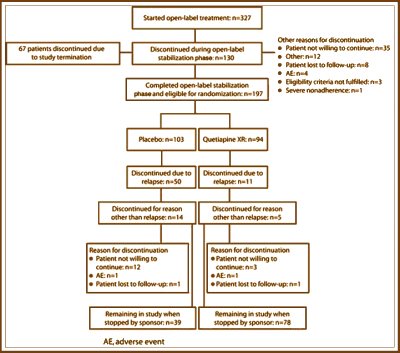
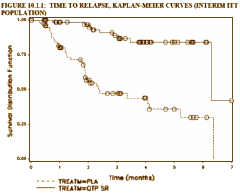
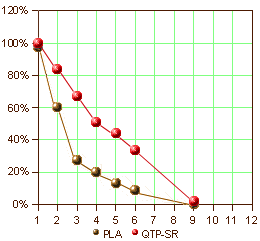
Confused? I was. It’s an odd study. 327 Stable subjects were changed over from their current medication to Seroquel XR and maintained for 4 months. Well actually, 260 subjects, since 67 of them didn’t make it to "randomization." That means that of those 260, 63 subjects left the study. So there was a 25% drop-out rate on Seroquel XR prior to "randomization." The circles on the graph above left shows where each of the subjects was at the time the study was stopped. The graph on the right is how far into the study subjects were at the time the study was terminated. Why change subjects to Seroquel XR for four months maintenance before starting a study to evaluate Seroquel XR as a maintenance treatment? Why not divide the group into three parts – continue current meds group, Seroquel XR group, Placebo group? Or just do the four days switchover to a Seroquel XR group and a Placebo group? It feels like they got their Seroquel XR dropper outers out of the way up-front. Another way to say that is that they selected Seroquel XR responders to see if they got a Seroquel XRresponse. It’s very odd, that’s all.
Seroquel XR: Bipolar Disorders
- NDA 22-047 S-006: monotherapy in the treatment of bipolar depression
- NDA 22-047 S-007: monotherapy in the treatment of bipolar mania
- NDA 22-047 S-008: adjunctive therapy in the treatment of bipolar mania
Effectiveness of the extended release formulation of quetiapine as monotherapy for the treatment of acute bipolar depression
by Trisha Suppes, Catherine Datto, Margaret Minkwitz, Arvid Nordenhem, Chris Walker and Denis Darko
Journal of Affective Disorders Volume 121, Issues 1-2, February 2010, Pages 106-115.This study was supported by AstraZeneca Pharmaceuticals LP, Wilmington, Delaware, USA (Study D144CC00002). Dr. Suppes has received funding or medications for clinical studies in the last 12 months from: Abbott, AstraZeneca, GlaxoSmithKline, JDS, National Institute of Mental Health, Novartis, Pfizer, and The Stanley Medical Research Institute. Dr. Suppes has had one advisory board with Orexigen and no speaking bureau activity within the last 12 months and has no conflicting financial interests or stock ownership. Royalties are received from Compact Clinical Publishers. We thank Jaya Gagwani, M.S., and Aruna Seth, Ph.D. from PAREXEL, who provided medical writing and editing support funded by AstraZeneca.
Seroquel XR: Major Depressive Disorder
Extended-release quetiapine fumarate [quetiapine XR] as adjunctive therapy in major depressive disorder [MDD] in patients with an inadequate response to ongoing antidepressant treatment: a multicentre, randomized,double-blind, placebo-controlled study
by Nizar El-Khalili, Mark Joyce, Sarah Atkinson, Robert J. Buynak, Catherine Datto, Petter Lindgren and Hans Eriksson
International Journal of Neuropsychopharmacology (2010), 13, 917–932.Abstract:
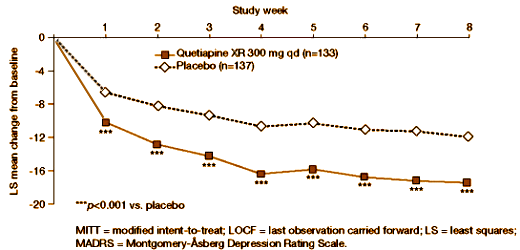
This study evaluated once-daily extended-release quetiapine fumarate (quetiapine XR) as adjunctive therapy in patients with major depressive disorder (MDD) with inadequate response to ongoing antidepressant treatment. In this 8-wk (6-wk active treatment/2-wk post-treatment drug-discontinuation/follow-up), multicentre, double-blind, placebo-controlled, Phase III study, 446 patients were randomized to quetiapine XR 150 mg/d, 300 mg/d, or placebo adjunct to ongoing antidepressant treatment. The primary endpoint was the change from randomization to week 6 in Montgomery-Asberg Depression Rating Scale (MADRS) total score. At week 6, MADRS total scores significantly improved with quetiapine XR 300 mg/d vs. placebo (-14.7 vs. -11.7, p<0.01). Quetiapine XR 300 mg/d showed significant improvements vs. placebo for: MADRS total score from week 1 onwards; MADRS response [(> or = 50% total score reduction) 58.9% vs. 46.2%, p<0.05] and remission [(total score < or = 8 ) 42.5% vs. 24.5%, p<0.01] rates; Hamilton Depression Rating Scale (HAMD) (-13.53 vs. -10.80, p<0.01) and Clinical Global Impression-Severity of illness (CGI-S) change (-1.52 vs. -1.23, p<0.05) at week 6. For quetiapine XR 150 mg/d, improvements were not significantly different vs. placebo, except for MADRS (weeks 1 and 2) and HAMD (week 6) total scores. Withdrawal rates due to adverse events (AEs) were: quetiapine XR 150 mg/d 11.5%, 300 mg/d 19.5%, and placebo 0.7%. The most common AEs (>10%) with quetiapine XR were dry mouth, somnolence, sedation, dizziness, constipation, nausea, insomnia, headache, and fatigue. In this study, quetiapine XR 300 mg/d as adjunctive therapy in patients with MDD with an inadequate response to ongoing antidepressant treatment was effective at week 6. However, the difference from placebo for quetiapine XR 150 mg/d at week 6 was not statistically significant. Both doses studied (150 and 300 mg/d) were effective at week 1 and generally well tolerated.
Statement of Interest: Nizar El-Khalili has received research grants from AstraZeneca, Eli Lilly, GlaxoSmithKline, Pfizer, and Sanofi-aventis, and has participated in advisory boards for Eli Lilly and speakers’ bureaux for AstraZeneca, Eli Lilly, and Sanofi-aventis. Mark Joyce has worked on clinical trials for the following companies: Abbott, Addrenex Pharma, Allergan, Allon Therapeutics, AstraZeneca, Avera, Bristol–Myers Squibb, Cephalon, DOV, Eli Lilly, Epix Pharmaceuticals, Forest, GlaxoSmithKline, Labopharm, Merck, McNeil, Myriad, Neurocrine, New River Pharmaceuticals, Novartis, Ono Pharma USA, Organon, Pfizer, Sanofi, Sepracor, Shire, Solvay, Somaxon, TransTech, and Wyeth. Sarah Atkinson has received research support from: Eli Lilly, AstraZeneca, Bristol–Myers Squibb, Insys, Lundbeck, Pfizer, Shire Pharmaceuticals, Sanofi-aventis, and Takeda Pharmaceuticals. Robert Buynak has received research support from AstraZeneca. Catherine Datto, Petter Lindgren, and Hans Eriksson are employees of AstraZeneca.Acknowledgements: This study was funded by AstraZeneca, manufacturer of quetiapine XR. We thank Dr Kim Croskery from Complete Medical Communications, who provided medical writing support funded by AstraZeneca.
This study deserves our close scrutiny. I have already reported on a sister study using Seroquel XR as a monotherapy in MDD [Extended Release Quetiapine Fumarate Monotherapy for Major Depressive Disorder: Results of a Double-Blind, Randomized, Placebo-Controlled Study, selling seroquel III: the data factories…, the clinical reasearch industry: the ‘atypical’ decade…]. That study was my paradigm for "Ghost-Busting," and it was, in my opinion, a dud. In that study, the Number Needed to Treat was included, "NNT values calculated using the Week 6 response data were: 8.1, 4.8, and 6.9 for quetiapine XR 50, 150, and 300 mg/day, respectively." I thought that was bad, but this study I’m describing seems worse. They took subjects who had not responded to antidepressants and added either Placebo or Seroquel XR in two doses – the famous atypical antipsychotic augmentation they’ve been muttering under their breath since Seroquel arrived on the market. Here’s their CRO-Chart:
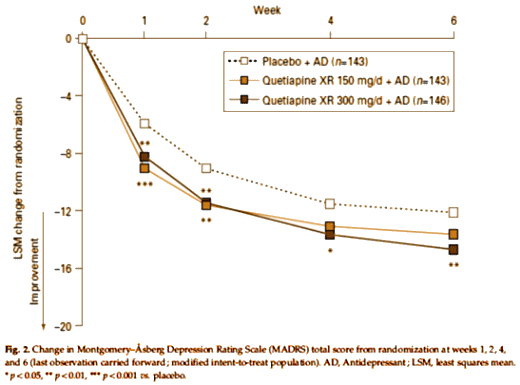
While achieving a little significance here and there, one could hardly claim that this was a successful trial, certainly not a robust response – closer to none at all. The Number Needed to Treat values were likewise pretty bad, "Using these MADRS response data, NNT values for quetiapine XR 150 mg/d and 300 mg/d were 18 and 8, respectively." NNT is the number of patients you need to treat to get one that beats a placebo. Low odds on success at the very best.
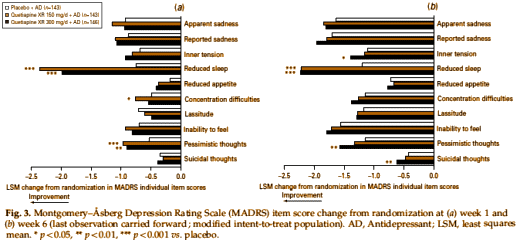
These are the individual changes in MADRS scores at week one and week six. The only significance is in sleep, and maybe in pessimism and suicidal thoughts. My interpretation of this study is that it shows how getting a good night’s sleep is really good for depressed people. Getting a good night’s sleep is one of Seroquel‘s strong points. There are plenty of other [cheaper][safer] sleeping pills. I see nothing here that says Seroquel XR is an antidepressant – only that it makes some depressed people feel a little better and sleep better.
 There are other trials of Seroquel XR in MDD, but the results are no more encouraging [link to pubmed]. If you look at those advertisements up top, they don’t say "Major Depressive Disorder," they say "depression." That means that the average reader is seeing that as saying if a patient says an SSRI didn’t help, Seroquel XR is the next thing to try. There’s nothing here to support that idea except that the FDA drank the Kool-ade. This is marketing, not medicine – certainly not evidence-based medicine. In spite of all the penalties and lost lawsuits, AstraZeneca is mounting a massive campaign to get people prescribing a medication with trivial [if any] effectiveness in depression. The only thing proven is its toxicity…
There are other trials of Seroquel XR in MDD, but the results are no more encouraging [link to pubmed]. If you look at those advertisements up top, they don’t say "Major Depressive Disorder," they say "depression." That means that the average reader is seeing that as saying if a patient says an SSRI didn’t help, Seroquel XR is the next thing to try. There’s nothing here to support that idea except that the FDA drank the Kool-ade. This is marketing, not medicine – certainly not evidence-based medicine. In spite of all the penalties and lost lawsuits, AstraZeneca is mounting a massive campaign to get people prescribing a medication with trivial [if any] effectiveness in depression. The only thing proven is its toxicity…
“My interpretation of this study is that it shows how getting a good night’s sleep is really good for depressed people.â€
Madhukar Trivedi, a university professor and consultant to AstraZeneca, came to the same conclusion in 2007 after reviewing data on the use of Seroquel for “TRDâ€. However he didn’t stop there. He was quite optimistic about the potential benefits of Seroquel in the treatment of “TRDâ€. See
http://tinyurl.com/4bb6f6n
At that time, Seroquel XR was in AstraZeneca’s pipeline.
http://tinyurl.com/4634h28
Subsequently, Seroquel XR was approved as an add-on treatment for “TRDâ€. Some of the regulatory documents are posted here:
http://tinyurl.com/688cm
Shortly thereafter, AstraZeneca was dinged for ‘overstating the efficacy of Seroquel XR and omitting material facts and risks associated with the drug.’
http://tinyurl.com/68gk9xd
I’ll have more to say about this after I get a good night’s sleep.