 Some sayings are so descriptive that they come to be a part of the language independent of their origins. The "elephant in the living room" is an Al-Anon saying to describe how some families react to having an alcoholic family member. It’s the major problem in the family, but it’s never discussed. They talk about mom’s depression, and the kids’ acting out, but not that dad gets drunk every night. The phrase has come to mean any very big thing that just isn’t open for acknowledgement and discussion. I keep thinking about that phrase as I read through these Clinical Trials of Psychiatric Drugs.
Some sayings are so descriptive that they come to be a part of the language independent of their origins. The "elephant in the living room" is an Al-Anon saying to describe how some families react to having an alcoholic family member. It’s the major problem in the family, but it’s never discussed. They talk about mom’s depression, and the kids’ acting out, but not that dad gets drunk every night. The phrase has come to mean any very big thing that just isn’t open for acknowledgement and discussion. I keep thinking about that phrase as I read through these Clinical Trials of Psychiatric Drugs.
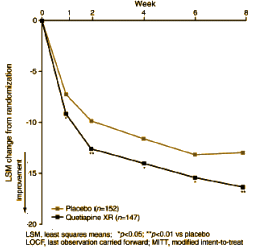
On the right is a typical graph from one of these studies [I call them
CRO-Charts]. What are the things not being talked about?
• The raw data doesn’t show. There’s no way to see if mean is in the middle of a tightly grouped set of observational data or values that are all over the place. Even though the Stardard Deviations are sometimes shown in the tables, they’re only recorded for the baseline and final periods, not the ones in between.
• There are high drop-out rates in these clinical trials. The drop-outs are corrected by the Last Observation Carried forward method [LOCF][see
contents of an empty mind…] – a method without rationale that assumes random drop-outs, but the time-line and reason for drop-outs isn’t included. It’s unlikely in a drug vs. placebo drug trial in sick people that the reason for dropping out is random.
• Frequently, the major change over the time in the study is the improvement on placebos, as it is here – a "drooping placebo" curve. This graph is from a study of subjects with Major Depressive Disorder. An improvement like that without treatment would be unprecedented. In a
recent study of duration in MDD, the mean duration of episodes was around 15 weeks:
But in these Clinical Trials, these great responses to placebos also occur in disorders like Schizophrenia or Mania – impossible.
• The modern Clinical Trial Industry doesn’t gather spontaneously appearing patients for their studies. They are "recruited," so one always wonder where they come from and how many actually have the disorders being studied. The source of subjects isn’t usually mentioned [see
the clinical research industry: the CRCs…].
These are not small things – obfuscated data, corrections with no way to determine if they are justified, phenomenal placebo improvements in disorders that don’t spontaneously improve quickly, and an unmentioned source of subjects without diagnostic documentation. That’s a lot. One might call these studies "faith studies" – and one is putting one’s faith in the company who pays for the study, hires the investigators, analyzes the results, and pays the actual writers – a company that stands to profit royally.
When I was reviewing the
Seroquel XR studies, I ran across a new "elephant" in one of the studies using the drug to treat Major Depressive Disorder that I thought I might pass along. It’s
the study where I got that
CRO-Chart in the upper right. Here’s what they actually did in that study:
METHODS: In this 10-week, (8-week active treatment phase and 2-week drug-discontinuation/tapering phase), multicenter, parallel-group, placebo-controlled, double-blind, randomized, Phase III study (D1448C00003: Opal), patients initially received quetiapine XR 150 mg/day or placebo. At Week 2, inadequate responders (<20% reduction in MADRS total score) were up-titrated to 300 mg/day quetiapine XR or matching placebo for the final 6 weeks. Primary endpoint: change from randomization to Week 8 in MADRS total score. Secondary endpoints included: MADRS response (≥50% reduction in total score from randomization) and changes from randomization to Week 8 in HAM-D and CGI-S…
LIMITATIONS:The study was not designed to compare quetiapine XR 150 mg/day and 300 mg/day; it was intended to reflect dose titration that might occur in clinical practice.
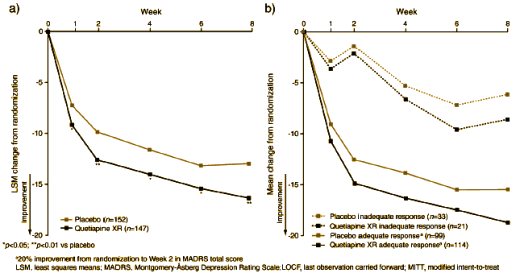
So they started with two groups – Placebo and Seroquel XR 150mg. After two weeks, they doubled the dose in all the subjects that didn’t respond. So now there are four groups for the last six weeks.
-
Placebo adequate responders [n=99]
-
Seroquel XR adequate responders [n=114][150mg/day]
-
Placebo inadequate responders [n=33][double placebo]
-
Seroquel XR inadequate responders [n=21][300mg/day]
A double placebo dose, you ask? That’s what it says:
Quetiapine XR and placebo were administered once daily, orally and in the evening. All patients randomized to quetiapine XR received 50 mg/day on Day 1 and were increased to 150 mg/day on Day 3 for the remainder of the first 2 weeks of treatment. Following 2 weeks of randomized treatment, patients with an inadequate response (defined as failure to achieve a ≥20% improvement compared with baseline in Montgomery-Åsberg Depression Rating Scale [MADRS] total score (Montgomery and Åsberg, 1979)) had their doses increased to twice their original dose (300 mg quetiapine XR or matching placebo). Those who exhibited an adequate response remained on quetiapine XR 150 mg/day for the remainder of the study. Investigators were blinded to the criterion defining inadequate response and were blinded to dose increase.
Here are the results. On the left, the results overall – a). On the right, the results broken down between the inadequate and adequate responders – b):
Look at the top line on the right hand graph. The inadequate Placebo responders responded to a double dose of Placebos. I find that truly remarkable! Since everybody was blinded, there was no way for the subjects or the investigators to know that they’d flunked and had gotten a double dose of Placebo, it must be a true scientific finding. To my knowledge, this is the first ever dose response curve for Placebos. Remarkable!
Of course, I’m being facetious. I can’t imagine any rational explanation for such a response. It’s just one more fishy thing about these endless Clinical Trials done on recruited subjects by compromised investigators with heavily massaged data analyzed and written by the Sponsors under the banner of evidence-based medicine [and by the way, the response to Seroquel XR in this study is as lousy as in the other ones]…
Role of funding source: Funding for this study was provided by AstraZeneca; AstraZeneca was involved in the development of the study design and in the analysis and interpretation of data; AstraZeneca provided funding for the writing of this manuscript.
Conflict of interest: Dr Brian Bortnick has no conflicts of interest. Dr Nizar El-Khalili has received research grants from AstraZeneca, Eli Lilly and Company, GlaxoSmithKline, Pfizer, Sanofi Aventis, and Takeda; and is a member of the speakers/advisory boards for AstraZeneca, Eli Lilly and Company, Sanofi Aventis, and Takeda. Dr Michael Banov is a member of the speakers/advisory boards for AstraZeneca, Bristol-Myers Squibb, Eli Lilly and Company, Forest, and Wyeth. Dr David Adson has received research support from Alkermes, AstraZeneca, BMS, Cyberonics, Eli Lilly and Company, GlaxoSmithKline, Novartis, and Pfizer; and is a member of the speakers/advisory boards for AstraZeneca, BMS, Forest, and Pfizer. Catherine Datto, Shane Raines, Willie Earley, and Hans Eriksson are employees of AstraZeneca.
Acknowledgements: This study (D1448C00003 [Opal]) was sponsored by AstraZeneca Pharmaceuticals. We thank Alex Mitchell, PhD, from Complete Medical Communications, who provided medical writing support funded by AstraZeneca.
 Some sayings are so descriptive that they come to be a part of the language independent of their origins. The "elephant in the living room" is an Al-Anon saying to describe how some families react to having an alcoholic family member. It’s the major problem in the family, but it’s never discussed. They talk about mom’s depression, and the kids’ acting out, but not that dad gets drunk every night. The phrase has come to mean any very big thing that just isn’t open for acknowledgement and discussion. I keep thinking about that phrase as I read through these Clinical Trials of Psychiatric Drugs.
Some sayings are so descriptive that they come to be a part of the language independent of their origins. The "elephant in the living room" is an Al-Anon saying to describe how some families react to having an alcoholic family member. It’s the major problem in the family, but it’s never discussed. They talk about mom’s depression, and the kids’ acting out, but not that dad gets drunk every night. The phrase has come to mean any very big thing that just isn’t open for acknowledgement and discussion. I keep thinking about that phrase as I read through these Clinical Trials of Psychiatric Drugs. On the right is a typical graph from one of these studies [I call them CRO-Charts]. What are the things not being talked about?
On the right is a typical graph from one of these studies [I call them CRO-Charts]. What are the things not being talked about?
Another “elephant” in the room is the university president who serves on the board of the drugmaker found guilty of fraud and continues to work like nothing happened; or the university professor who serves as a consultant to the drugmaker found guilty of fraud and continues to conduct business as usual.