There were two identical clinical trials of Abilify as an adjunct in the treatment of Major Depressive Disorder considered by the FDA in their approval evaluation. Subjects with a past history of poor responses to antidepressants during previous episodes were treated for eight weeks with a variety of SSRIs. Non-responders were then randomized and treated with either Placebo or Abilify for six weeks.
| The Protocol
|
|
The study phases are outlined as follows:
• Phase A: Screening for eligibility in which MDD patients how had less than 50% improvement on past ADT [as perceived by the patient] using criteria specified later.
• Phase B: An 8-week phase of single-blind [SB] placebo coadministered with openlabel [OL] treatment of 1 of 5 ADTs [escitalopram, sertraline, venlafaxine extendedrelease, fluoxetine or paroxetine controlled-release]. This phase allowed for a prospective identification of inadequate responders as defined by meeting the following criteria during Phase B:
• Had <50% improvement on the Hamilton Depression Rating Scale [HAMD17]
• Had a HAMD7 score of at least 14 units
• Had a Clinical Global Impression [CGI] score of no better than minimal improvement
• Phase C: A 6-week phase in which inadequate responders were randomized to DB placebo or aripiprazole [flexible dose] while continuing their OL ADT [at the same dose received during Phase B of the study]. The flexible daily dose range of aripiprazole treatment was either:
• 2 to 15 mg daily in subjects receiving ADT of a potent CYP2D6 inhibitor [fluoxetine or paroxetine].
• 2 to 20 mg daily in subjects receiving other ADTs.
|
These are the results that got them approved [from the FDA review document][LOCF method for missing values]:
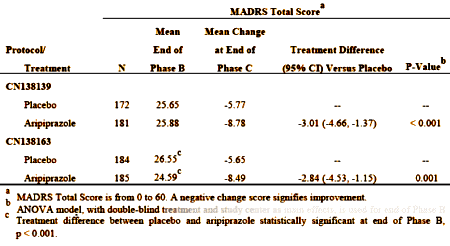
This is the overall graph from CN138163 [the second trial]:
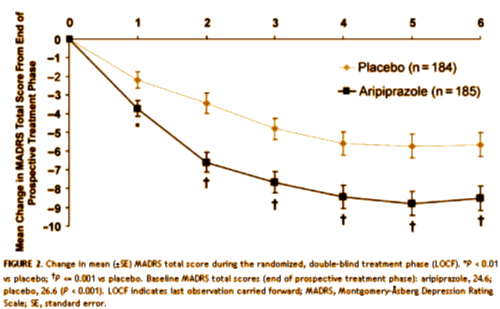
But there was a problem in CN138163 [not mentioned in the published study but noted in the FDA report]:
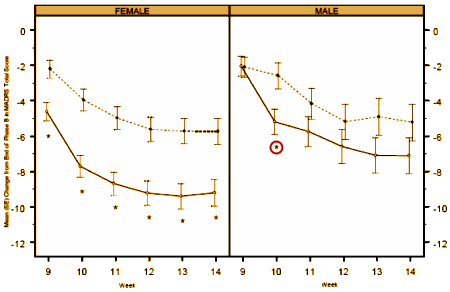
The difference was even more marked in the earlier study [CN138139]:
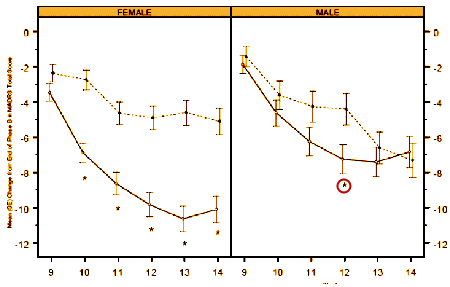
The response was more marked and significant in females – not so much in males [lonely p values for males in both studies].
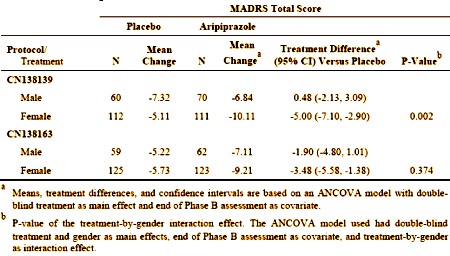
The report comments on but doesn’t explain this difference, and it seemed to have had no bearing on the approval.
The MADRS scale was not the only instrument measured. There were two that were subject self ratings [IDS-SR and QIDS-SR] shown here from the study that was available to me [CN138163].:
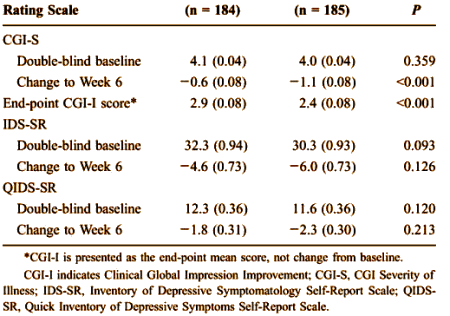
Apparently, the subjects themselves were less taken with the response than the authors, a finding rationalized away in the article:
Here’s a side-by-side comparison of the Abilify and Seroquel XR MADRS graphs for atypical antipsychotic augmentation in Major Depression [adjusted to the same scales]. I’ll defer my comments after a bit of cogitation:
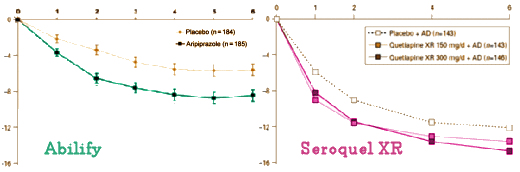
Have you ever seen this http://www.propublica.org/projects/reinstein/docs/reinstein.depo.seroquel.suit.opt.pdf
Michael Reinstein’s depo –he was paid boatloads from AZ and is known as the ‘Clozaril King’ as well.
Yikes! I’d read about the Seroquel, but not the Clozapine. What a dangerous guy!