This blog came by its name honestly. It started as 3oldmen.com, intended to be the blog of three of us retirees, but the other two didn’t go for the idea as much as they thought they might. But then I got going on the deceit of the Bush Administration in getting us involved in the Iraq war, and my daughter, in a heartfelt attempt to tell me that my endless research into those details was not interesting, said, "you should rename it 1boringoldman.com." Since I was more interested in what I was interested in than in being interesting, I did [rename it].
I fear I’m on a similar tach right now – boring details. What I’m interested in is figuring out when and how this business of clinical trials in the pharmaceutical industry became so incredibly corrupt. And how academic medicine, and Psychiatry in the specific, got so tangled up in the whole business. So I’m edging my way backwards through the FDA approvals of the Atypical Antipsychotics, looking for signs of the times when science was science, not marketing. In this post, I’m looking at the original FDA Approval of Eli Lilly’s Zyprexa [Olanzapine]. What I know is that Eli Lilly was a big offender and was later fined $1.4 B for false advertising and sleazy marketing practices related to Zyprexa. So I wanted to look at the beginning, to see if the Zyprexa story was corrupt from the very start. I haven’t read the "Zyprexia Documents" yet. I wanted to look at the original before seeing the part that is sure to enrage…
Eli Lilly’s Zyprexa [Olanzapine] was approved by the FDA for treatment of Schizophrenia on September 30, 1996. The FDA Review considered four multicenter trials: HGAP [12 US], HGAD [23 US and Canada], E003 [50 Europe, South Africa, Isreal, and Australia], and HGAJ [186 US and Europe].Study E003 was a failed study with multiple doses of Olanzapine and Haldol. Nothing achieved significance and the study was administratively flawed. Study HGAJ was a head to head Olanzapine vs Haldol long term trial. Although Olanzapine was superior, the reviewers ruled that the design was biased. So approval actually rested on HGAP and HGAD.
Trial HGAP
This was a simple double-blind Placebo vs Olanzapine 1mg/day vs Olanzapine 10mg/day trial. The results [from the FDA Review tables]:
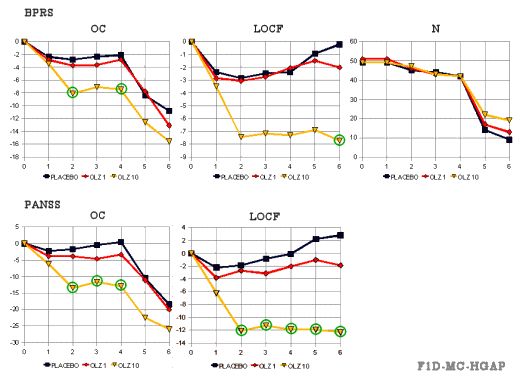
HGAP was published. The observed case [OC] graphs were published as shown below [no LOCF graphs!]. Their explanation for the reason for the big drop-out at week 4 was that they let the non-responders out of the acute phase and up-ed their meds [OLZ 20mg/day] at that point [plausible]:
colored [by me] for clarity
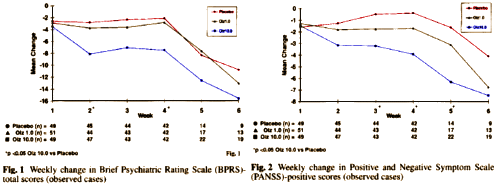
Olanzapine versus placebo: results of a double-blind, fixed-dose olanzapine trial
by Charles M. Beasley Jr, Todd Sanger, Winston Satterlee,Gary Tollefson, Pierre Tran, Susan Hamilton – Olanzapine HGAP Study Group
Psychopharmacology (1996) 124:159-167.This study was funded by Eli Lilly and Company. C.M. Beasley Jr, E Sanger, W. Satterlee, G. Tollefson, R Tran, S. Hamilton Psychopharmacology Division, Lilly Research Laboratories. Acknowledgements: The authors thank Kim Musick, Jan Hanshew, Patrice Bradley, and Amy Kuntz for assistance in study administration and manuscript preparation, as well as AnnMarie Crawford, Mary Anne Dellva, and Roy Tamura for additional statistical support.
Dr. Beasley’s internship was in the Department of Psychiatry, Yale University and he completed his training in psychiatry at the University of Cincinnati in 1987. He immediately joined Eli Lilly and Company in the area of clinical development of psychiatric medications. He was responsible for Prozac from the time of its US launch through 1991 working extensively on the topic of the potential for SSRI medications to induce suicidality. During this period of time he was also responsible for the development program for atomxetine as an antidepressant [program terminated)]during which he developed a particular interest in placebo response and trial design. From 1991 through 2001 he was responsible for the development of Zyprexa as a treatment for schizophrenia…
Trial HGAD
This one was more complex. It compared Placebo vs Olanzapine in three dose ranges vs Haldol. The results [from the FDA Review tables][the right graph is the drop-outs]:
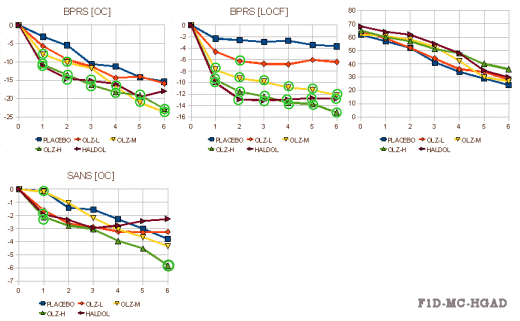
Olanzapine versus placebo and haloperidol: acute phase results of the North American double-blind olanzapine trial
by Beasley CM Jr, Tollefson G, Tran P, Satterlee W, Sanger T, Hamilton S., and the Olanzapine Study Group
Neuropsychopharmacology. 1996 Feb;14(2):111-23.
From the Psychopharmacology Division, Lilly Research Laboratories, Eli Lilly and Company. Acknowledgments: The authors thank Nancy C. Andresen,MD, PhD, Andrew H. Woods Professor of Psychiatry, University of Iowa College of Medicine, Iowa City, IA, for assistance in planning the study, providing rating scale training, and serving as principal investigator; Jan Hanshew, Marcia Nordman, Mindy Davis, MTSC, Mary Ann Heath, Carol Mattler, Pat Cowell-Hanover, PhD, Marie Reamer, Ray Albritton, Tom Voegele, and Charlotte Luttman for assistance in study administration and manuscript preparation.

Trial HGAJ
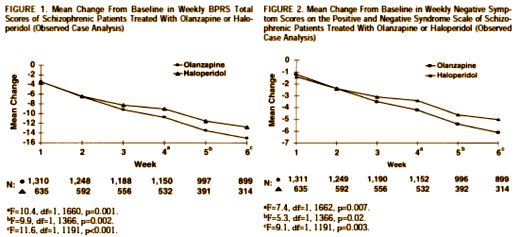
Olanzapine versus haloperidol in the treatment of schizophrenia and schizoaffective and schizophreniform disorders: results of an international collaborative trial
by Tollefson GD, Beasley CM Jr, Tran PV, Street JS, Krueger JA, Tamura RN, Graffeo KA, Thieme ME.
American Journal of Psychiatry. 1997 Apr;154(4):457-65.From Lilly Research Laboratories. Address reprint requests to Dr. Tollefson, Lilly Research Laboratories, Eli Lilly and Company, Lilly Corporate Center, Drop Code 0538, Indianapolis, IN 46285. The authors thank Suzanne Myers for technical and editorial assistance in the preparation of this manuscript.
Objective: This international, multicenter double-blind trial was designed to compare the therapeutic profile of an atypical antipsychotic, olanzapine, with that of a conventional dopamine D2 antagonist, haloperidol.Method: A total of 1,996 patients at 174 sites in Europe and North America were randomly assigned to treatment with olanzapine (N=1,336) or haloperidol (N=660) over 6 weeks. The primary efficacy analysis involved the mean change from baseline to endpoint in total scores on the Brief Psychiatric Rating Scale (BPRS). Secondary analyses included comparisons of the mean change in positive and negative symptoms, comorbid depression, extrapyramidal symptoms, and overall drug safety.Results: Olanzapine demonstrated clinical results superior to those of haloperidol on overall improvement according to the BPRS and on every secondary measure, including depression. Olanzapine was also associated with significantly fewer discontinuations of treatment due to lack of drug efficacy or adverse events. Substantially more olanzapine-treated patients (66.5%) than haloperidol treated patients (46.8%) completed 6 weeks of therapy. Statistically significant advantages of olanzapine treatment were related to 1) change in negative symptoms, 2) extrapyramidal symptom profile, 3) effect on prolactin levels, and 4) response rate.Conclusions: Olanzapine shows a superior and broader spectrum of efficacy in the treatment of schizophrenic psychopathology, with a substantially more favorable safety profile, than haloperidol. It meets several of the criteria for a novel atypical antipsychotic agent.

Weight Gain
Because weight gain would become such an issue later, I’ve included the data from the review and the discussion of labeling [the weight gain in the second table is % with >7% weight gain].
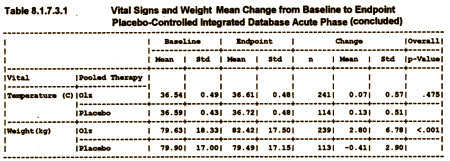
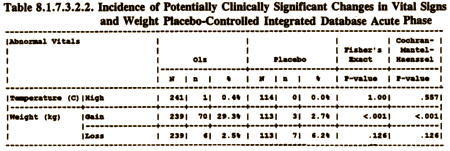

Then from the negotiations about labeling after approval:

In 1996, Zyprexa was the new kid on the block, following Risperdal. I was frankly a little surprised at what I read here. These articles were industry generated studies. That’s how they were clearly labeled in the literature. They didn’t stick some academic psychiatrist on as first author like the Seroquel articles I reported on earlier. They acknowledged support staff, including writing support, but that didn’t bother me as much as it did in the Seroquel and Abilify articles, because they were up-front about who did the study. I was glad to see the "observed cases" reported. They didn’t hide behind those LOCF CRO-Charts, instead letting us see what they saw. They reported the weight gain in the FDA and published information instead of using all the stealth techniques employed by AstraZeneca with Seroquel. I’m not trying to say Lilly was the paradigm of scientific purity here, but they were good enough for me on this earliest foray.
Lilly Zyprexa can cause diabetes I took Zyprexa a powerful Lilly schizophrenic drug for 4 years it was prescribed to me off-label for post traumatic stress disorder was ineffective costly and gave me diabetes.
Please take with caution and learn as much as you can about side effects. Eli Lilly’s #1 cash cow Zyprexa drug sale $40 billion dollars so far,has a ten times greater risk of causing type 2 diabetes over the non-user of Zyprexa. So,here we have a conflict of interest that this same company also is a big profiteer of diabetes treatment.
FIVE at FIVE
The Zyprexa antipsychotic drug,whose side effects can include weight gain and diabetes, was sold for “children in foster care, people who have trouble sleeping, elderly in nursing homes.”
Five at Five was the Zyprexa sales rep slogan, meaning 5mg dispensed at 5pm would keep patients quiet.
– Daniel Haszard Zyprexa Whistle-blower
Thank goodness for your daughter, the details here are in no way ‘boring’ and your input and thoughts behind the stats are a welcome and necessary commentary.
Zyprexa chronicles…this is the as in *the* drug that caused the most chemical harm to my daughter. Many docs are at Furious Seasons waiting for you to read. I have anecdotal stories abt antidepressant molecular chain connection that mimics antidepressant reaction (negative) w this drug, the massive weight gain and metabolic syndrome associated as well as the ocular dysfunction that created a living nightmare for my child as this drug was rx to an underage 18 child age 11 thru 17 and again at age 18 in 40mg doses by an inpatient doctor. the results are what some ppl have pondered: “stroke victim” or zyprexa victim?. thanks doc for this blog, it will help others.
You are right Stephany!…details not boring at all !
One more brillant article.
http://doctick.com/medical_calculator/files/BPRS-24-brief-psychiatric-rating-scale.html
“What I’m interested in is figuring out when and how this business of clinical trials in the pharmaceutical industry became so incredibly corrupt. And how academic medicine, and Psychiatry in the specific, got so tangled up in the whole business. ”
It’s the unholy triumvirate of greed, gullibility, and ego. Pharma was stating publicly over a decade ago that it is primarily a marketing concern. They and their cohorts (ad agencies, CROs, IRBs) are about making money. That’s it. Gullibility and ego appiy to both docs and patients. Patients want to feel they’re informed, and will reject any information that makes them feel less than expert. With docs, it’s a little more complicated. Many resent that they are not making more money. They want to be “in the know”–this is what drove participation in speakers’ bureaus, acceptance of invites to resorts, etc. There is also a widespread feeling that if others do it, why not them. And many believe that they are not susceptible to marketing messages.
There’s a lot more but those are the basic themes. Changing the landscape means getting people to believe they’ve been duped. Ain’t gonna happen. And meanwhile pharma will make money any way it can.
“. . . I was more interested in what I was interested in than in being interesting . . . .â€
I’m glad you are. While I can’t be interested in everything that interests you, there’s almost always enough overlap for me to learn something from your reflections. And in matters about which I am essentially ignorant – your recent discourses on pharmaceutical corruption, for instance – I find it interesting in spite of myself. This is good stuff! Who knew? Just keep it up, please, and thanks.
I concur with Woody! He said it better than I would have so I’ll just go with “ditto”.
Daniel brings up Viva!Zyprexa! campaign that sold doctors on giving the Zyprexa 5mg at 5pm and that was sadly my daughter’s “5 oclock pill” , which means her doctor heard from a drug rep. when she gained over 100 lbs the doctor told her to “take a walk, dont drink soda”. just like the campaign said to do. she DID walk and she never drank soda, the weight dropped off once the drug was removed, and now she just can’t think or read or function in this world and is disabled.
Thanks Lilly, you’re worse than AstraZeneca. Maybe it’s a tie.
After reading some of the comments above I don’t think being interesting all of the time is so important. There are a lot of people who wish someone cared enough to get to the bottom of this web of deceit a long time ago before these drug companies seriously hurt many patients trying their best to get well. there should be consequences for their actions.