Zyprexa [Olanzapine] is a solid antipsychotic, effective in the treatment of Schizophrenia. There’s never been a question about that, so it was an early market success. But, as mentioned in the last post,
Lilly was after more than the limited Schizophrenia market and had come up with the strategy to market it to Primary Care Physicians to treat
symptoms and behaviors. Then the weight gain became apparent and reports of adverse events began to show treatment emergent Diabetes [from the
legal documents]:
While Lilly’s marketing efforts were highly successful, the numbers of adverse event reports submitted to the FDA and made available to Lilly through the MedWatch database also steadily increased. For example, in one Periodic Adverse Drug Event Report, Lilly reported five instances of diabetic acidosis and two instances of diabetic coma between September 30 and December 30, 1997. Another report listed three incidents of ketoacidosis from April 1 to June 30, 1998. By the end of 1998, after two years on the market, the diabetes-related AERs for Zyprexa totaled nearly 200. By the end of 2000, that number was approximately 600.
By 2000, people were beginning to formally ask questions, specifically about Diabetes:
By 2000, European officials were expressing concern about the risks and side effects of Zyprexa. On February 21, 2000, the European Agency for the Evaluation of Medicinal Products ("EAEMP") contacted Eli Lilly Ltd. UK and ordered the company to expedite its review of risk factors and provide the information quickly… We would like to inform you that CPMP [the European Union’s Committee for Proprietary Medicinal Products] concluded that there have been several reports of diabetic ketoacidosis, some with fatal outcome and a cumulative review should be provided of all known or suspected cases as soon as possible… On May 1, 2000, the FDA requested more information from Lilly on Zyprexa’s relationship to hyperglycemia and diabetes.
| There come a point in any story where things can go down two differing paths. Back in 1974, that point was reached with Clozapine. In response to the reports of Agranulocytosis, the manufacturer withdrew the drug from the market. That point was reached in the summer of 2000 with Zyprexa, and Lilly chose a path: |
The clinical meaning of the weight gain is difficult to assess, since in the experience of the investigator over 20 years, patients generally tend to gain weight while enrolled in studies at the Lilly Clinic. The reasons for weight gainmay be attributed to lack of exercise and liberal access to high fat meals… On the basis of [] case studies it appears as though patients that may develop hyperglycemia in temporal association with olanzapine are patients that are typically at risk for DM-II based on race, obesity, or family history. It is unclear at this point whether or not the number of cases of olanzapine in temporal association with DM-II exceeds the expected incidence for the development of DM-II in patients with schizophrenia. [Response to the FDA – July 31, 2000].
I’m not suggesting that they should have withdrawn
Zyprexa at that point. It’s a safer drug than
Clozapine, which is on the market. But nobody’s getting rich on
Clozapine because its downside is widely publicized.
Lilly could have explored the Diabetes issue and still had a useful and viable drug, but its use would be limited. They didn’t do that, but they
knew:
In October 2000, following a meeting with members of one of Lilly’s academic advisory boards, Lilly executives discussed the reactions of the board endocrinologists to the company’s data on Zyprexa and weight gain, hyperglycemia, and diabetes. "Unfortunately, this consultation reinforced my impression that hyperglycemia remains quite a threat for olanzapine and may merit increasing even further medical attention and marketing focus on the topic… They were however concerned by our spontaneous AE reports, and quite impressed by the magnitude of weight gain on olanzapine and implications for glucose…" [Dr. Robert Baker, Medical Advisor and Senior Clinical Research Physician]
In response, Dr. Thomas Brodie reiterated that "clearly, this group of Endocrinologists … are very concerned with the approach Lilly is taking towards the issue that Zyprexa [sic] leads to diabetes… I do believe they made a very strong point that unless we come clean on this, it could get much more serious than we might anticipate."
Dr. Charles Beasley who had headed the early Zyprexa effort and authored their first articles on the clinical trials responded, "There is the marketing approach and then the scientific analyses approach… There are 2 issues – weight gain and hyperglycemia. These guys were really concerned about the weight gain, not only because of a diabetes risk but all the other potential health risks… When they understood… that olanzapine is the worst offender, other than clozapine, they advocated a different marketing strategy than we are taking. They believe we should "aggressively face the issue" and work with physicians to address methods of reducing weight gain… There does not seem much to say about scientific analyses of weight gain, we know it’s a weighty problem. When you translate 1-2% gain of 40+ kilos into the absolute number based on 5 million patients, the number is 50,000 to 100,000. 100,000 people putting on 90 pounds of weight is a lot… With regard to the marketing side of this issue of impaired glucose tolerance [sic] / diabetes, the message was clear. Don’t get too aggressive about denial, blaming it on schizophrenia, or claiming no worse than other agents until we are sure of the facts and sure that we can convince regulators and academicians." [Email from Charles M. Beasley, Jr., to Alan Breier, Oct.10, 2000]
Lilly couldn’t deny the weight problem. They’d reported it in their own studies. But they continued to vigorously deny that
Zyprexa was associated with Diabetes. They
trained their sales force to deal with these complaints:


Following several months of study by the LillyUSA Zyprexa Brand Team, the affiliate approved the recommendation that Lilly actively promote Zyprexa to selected current primary care prescriber targets… We believe there to be significant unmet medical need among office-based primary care physicians… Zyprexa’s profile is ideal for primary care (safe, simple, well-tolerated, effective, versatile). Zyprexa would enjoy first mover advantage in this segment…
Challenges: Most PCPs currently prescribe a low volume of antipsychotics and mood stabilizers. Many PCPs will refer patients in need to psychotropic treatment to a specialist rather than treat the patient. Key barriers to uptake include PCP’s lack of training in this category, limited time with patients, and an aversion to perceived risk. Zyprexa’s primary indications – schizophrenia and bipolar -are not viewed as PCP-treated conditions, so there’s not a specific indication for Lilly reps to promote in the PCP segment…
Position: Zyprexa: The safe, proven solution in mood, thought, and behavioral disorders. We will emphasize safety to address barriers to adoption …. The word ‘solution’ speaks to unmet medical need, and enables the PCP to take control of clinical situations that previously had led to referrals and/or poor outcomes. ‘Mental disorders’ is intentionally broad and vague, providing latitude to frame the discussion around symptoms and behaviors rather than specific indications.
| So we’re back to Lilly’s path again. They ignored their own internal dialog about the Diabetes and weight gain issues and went down a very slippery slope. Rather than seeking a whole lot more indictations to advertise or stealthily promoting off-label prescribing like AstraZeneca did with Seroquel, they came up with something else even more devious. They decided to take prescribing Zyprexa out of the hands of Psychiatrists and put it in the hands of Primary Care Physicians. And further, they proposed to skip the pesky task of diagnosing Mental Illness altogether and get doctors using their antipsychotic drug to treat symptoms – like "suspiciousness." It’s a remarkably brash idea, taking medicine back to prehistory on the banks of the Amazon or the mountains of Peru. I’m not sure that the magnitude and arrogance of Lilly’s campaign has received its proper due. Instead of addressing the dangers of their drug, they proposed to use it as a routine symptomatic medication in a busy GP’s office practice. Truly remarkable… |
I think I’m going to wrap this post up and leave the what happened part for another session. I’m a Psychiatrist, so I suppose I’m vulnerable to the charge that the reason this particular piece is so upsetting to me is some kind of specialty narcissism – taking me and my guys out of the loop. That may be, but what I think I find so upsetting is something else. I spent the first part of my career as a Primary Care Physician [Internal Medicine]. The very idea that I would see a new Schizophrenic patient in my office and treat him for symptoms of "suspiciousness" strikes me as belonging in the theater of the absurd. No amount of "detailing" or brochure reading back then could’ve prepared me to adequately evaluate or treat the patients Lilly proposed I could handle with a prescription for Zyprexa. There’s little that might occur in the life of a young person that deserves more attention than a Schizophrenic Break, and such a situation requires all the expertise and experience a person can muster. There is just no such thing as a "routine" Schizophrenic Break or "routine" Mania – even for an old guy like me.
| Psychiatry and Psychiatrists take a lot of hits – some well deserved. But Lilly‘s deciding to reduce the major mental illnesses like Schizophrenia and Manic-Depressive Illness to symptoms treated in a Primary Care Physician’s office with an antipsychotic the likes of Zyprexa is no place to turn the other cheek. To me, this is incitement to malpractice. And to just blow off looking into their routine office visit medication as a potential cause of Diabetes is criminal negligence. It’s that simple… |


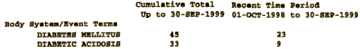
It makes sense to call it criminal negligence, that’s why the DOJ fine for corporate crime of illegal marketing of Zyprexa is the gateway to saying corporate crime and criminal negligence caused undue harm, body damage and limitless life change stress occurance to patients. Yet, as with Seroquel injured patients are not seeing one dime of restitution for a serious crime that happened to them as the result of a pharmaceutical company committing corporate crime against them.
If doctors became whistleblowers NOW each time they saw or see paperwork from drugs or drug rep detailing, they *could* help police the matter and become savvy soldiers in the crime against their patients and themselves, but everyone gets off the hook too easy. Think about Rebecca Riley, age 4 dead of psych med intoxication–her doctor took the stand under immunity and later settled for 2 million, got her license back and is practicing medicine again. The parents were accused of killing (murder) their child and are both in prison, the siblings in foster care, Rebecca dead, and the outside parties are all carrying on as usual.
That’s immorally and unethical wrong, and disgusting. Same as how after over a decade my daughter exists as a completely different person, lacking ability to live in the ‘real world’, missed high school graduation, no college, no friends…yet at age 17 she researched her own medication, Zyprexa, the one giving her the most problems, took the paperwork to her psychiatrist showing him the diabetes side effects, weight gain, eye issues, etc asked to go off of it and that guy told her to take a walk….she fired him. Bravo to her, that was unknown to us all to be her last self-advocacy she was able to do, as the withdrawals sent her into a spiral and the adding back of the drug by a quack doctor at over-dose amounts basically destroyed her brain. Life goes on, for all. Not her. What’s wrong with this picture?
Does anyone in the general public know about Viva!Zyprexa! 5 at 5? I doubt it. Well, that campaign is what ruined a young person’s life who is NOT schizophrenic. How about that for a crime?
I think I may have mentioned in some previous post that I consider Lilly the trendsetter in marketing . . . and it began BEFORE Zyprexa. When Lilly gained the rights for rDNA insulin, there was essentially no market. Diabetics who had used natural (animal-source) insulin for decades were certainly not clamoring for a change. The only possible beneficiaries of rDNA “human” insulin were those few who had allergic responses to beef, pork or beef-pork insulins . . . a very minuscule population. Nevertheless, seeking a hefty ROI, Lilly fear-mongered that the supply of animal pancreases was insufficient (an outright lie). They developed talking points: Why would humans want to use anything BUT ‘human’ insulin (with no proof that the brew they formulated was structurally identical to human insulin). They co-opted enough KOLs to change the recommendations emerging from our medical schools. In slightly more than a decade, the gold standard(s) of diabetes treatment had been demonized and labeled as ‘dirty.’ Patients who neither wanted or needed this new “latest and greatest” breakthrough discovery were forced to change treatment protocols or seek to import insulin from other countries (through a very laborious and problematic carve-out), or use a product that to this day has never been reviewed by FDA examiners. I continue to believe that Lilly’s successful marketing and influence-peddling regarding rDNA insulin laid the groundwork for the explosion of questionable marketing and medical detailing that has emerged since that time, not only for their own marginal products but for those competitors who saw how easily our regulators could be ‘handled’. When there is no accountability, no transparency, and no real penalty for wrongful behavior . . . you just get more wrongful behavior.
The number of diabetics who have been harmed or killed by rDNA insulin is unknown and unknowable since the very nature of diabetes and the patient-intensive management of the disease provide viable scapegoats for a criminal pharmaceutical enterprise.
Stephany,
That’s a very painful story. And there are lots of similarly painfully stories like yours out there – unredeemable stories. The shame of Lilly’s Zyprexa is that the drug was, in fact, an improvement over Clozapine. But that’s all it was – a Refractory Schizophrenia drug and there’s a place for one of those. There aren’t really any other indications that make any sense.
I’m sorry you and your daughter had to come along at the time when Zyprexa was being played with so carelessly. I’m glad you’ve decided to do what you can to prevent it from happening in the future. You’re good at it. I wish more people would follow your lead.