Atypical antipsychotic-induced diabetes mellitus: how strong is the evidence?
Henderson DC
CNS Drugs. 2002;16(2):77-89.
… Even more concerning, because of a significant risk of death, there have been numerous case reports of patients treated with clozapine or olanzapine developing diabetic ketoacidosis shortly after initiation of the drug. Much of the information concerning the medical morbidity of diabetes mellitus is based on case reports, retrospective chart reviews, naturalistic studies and cross-sectional studies. While definitive studies have yet to be reported, mounting evidence suggests that the atypical antipsychotic agents, particularly clozapine and olanzapine, may significantly impair glucose metabolism and increase the risk of diabetes in patients with schizophrenia. Diabetic ketoacidosis, although it appears to be uncommon, is of great concern secondary to the risk of death…
Consensus Development Conference on Antipsychotic Drugs and Obesity and Diabetes
American Diabetes Association, American Psychiatric Association, American Association of Clinical Endocrinologists, and North American Association for the Study of Obesity
Diabetes Care 27:596–601, 2004.
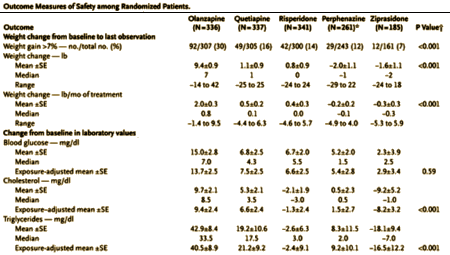
SUMMARYThe SGAs are of great benefit to a wide variety of people with psychiatric disorders. As with all drugs, SGAs are associated with undesirable side effects. One constellation of adverse effects is an increased risk for obesity, diabetes, and dyslipidemia. The etiology of the increased risk for metabolic abnormalities is uncertain, but their prevalence seems correlated to an increase in body weight often seen in patients taking an SGA. Direct drug effects on β-cell function and insulin action could also be involved, since there is insufficient information to rule out this possibility. In the general population, being overweight or obese also carries a much higher risk of diabetes and dyslipidemia.These three adverse conditions are closely linked, and their prevalence appears to differ depending on the SGA used. Clozapine and olanzapine are associated with the greatest weight gain and highest occurrence of diabetes and dyslipidemia. Risperidone and quetiapine appear to have intermediate effects. Aripiprozole and ziprasidone are associated with little or no significant weight gain, diabetes, or dyslipidemia, although they have not been used as extensively as the other agents.
Drug Weight Diabetes Lipids Clozapine +++ + + Olanzapine +++ + + Risperidone ++ D D Quetiapine ++ D D Aripiprazole * +/− − − Ziprasidone * +/− − − + = increase effect; − = no effect; D = discrepant results
* = Newer drugs with limited long-term data.
The choice of SGA for a specific patient depends on many factors. The likelihood of developing severe metabolic disease should also be an important consideration. When prescribing an SGA, a commitment to baseline screening and follow-up monitoring is essential in order to mitigate the likelihood of developing CVD, diabetes, or other diabetes complications.
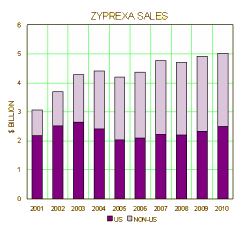
We might as well include the further data about weight gain that came along in 2005. The CATIE Trial, an NIMH trial comparing the Atypicals, reaffirmed Zyprexa‘s prowess as an antipsychotic,
but also definitively put Zyprexa at the top of the weight gain chart for all times.
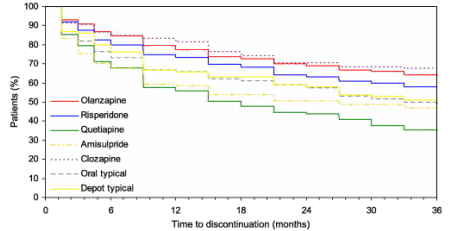
An exhaustive review by Newcomer in 2005 confirmed the ranking of different Atypical drugs suggested by the Consensus Development Conference and added an interesting piece of information – one fourth of the cases of Diabetes occurred in patients who had not gained weight, and the peak time of onset was in the early months of treatment [first three months]. These findings suggest that these drugs have some direct effect on glucose metabolism independent of the weight gain itself. Clozapine and Olanzapine were at the top of the list in this review.
 The scientific method has been with us for centuries. Observations lead to Questions; Questions to Conjecture; Conjecture to Experiments, Experiments to Results, and Results become New Observations leading to New Questions. There is no end point – just a great big circle that never ends. We venerate our famous scientists for their Conjectures, but it’s their ability to make New Observations of someone else’s Results that really matters. In ancient history, Galileo’s observations and his hypothesis that the earth moved around the Sun was opposed by the Catholic Church because it conflicted with the Bible – the source of absolute knowledge in that time. But in the case of Eli Lilly, their persistent denial of the "metabolic syndrome" of their blockbuster Zyprexa had a much more banal driver – corporate profit. Lilly set out to have the "number one neuroscience pharmaceutical in history," and in the process, they threw neuroscience to the wind.
The scientific method has been with us for centuries. Observations lead to Questions; Questions to Conjecture; Conjecture to Experiments, Experiments to Results, and Results become New Observations leading to New Questions. There is no end point – just a great big circle that never ends. We venerate our famous scientists for their Conjectures, but it’s their ability to make New Observations of someone else’s Results that really matters. In ancient history, Galileo’s observations and his hypothesis that the earth moved around the Sun was opposed by the Catholic Church because it conflicted with the Bible – the source of absolute knowledge in that time. But in the case of Eli Lilly, their persistent denial of the "metabolic syndrome" of their blockbuster Zyprexa had a much more banal driver – corporate profit. Lilly set out to have the "number one neuroscience pharmaceutical in history," and in the process, they threw neuroscience to the wind.
What’s easy to miss in this story is that Lilly threw out the neuroscience before the reports of the "metabolic syndrome" even began to emerge. Psychiatrists use potentially dangerous drugs to treat a dire illness – Schizophrenia. Lilly introduced this derivative of our most dangerous drug [Clozapine] and from early on promoted using it to treat a wide range of disorders whose severity didn’t justify even the known dangers of the medication. And further, they pushed removing it from the hands of the doctors who had at least seen these illnesses and the consequences of treatment [Tardive Dyskinesia] – encouraging General Practitioners to prescribe Zyprexa based on office visit symptoms and behaviors. Then Lilly tried to dismiss the "metabolic syndrome" even when it was visible to people on the street, and deny that their medicine was the leading cause.

“I doubt that Dr. Robert Baker, Dr. Charles Beasley, Dr. Gary Tollefson, Dr. Alan Breier, or any of the other higher-up Lilly doctors involved in the Zyprexa story ever planned to throw science out the window either when they were sitting in their pharmacology classes as young men in medical school. But that’s exactly what they did…”
Multiply this behavior thousands upon thousands of times; and you have a fairly well focused picture of the mental health system in America. The modern Corporate Pharmaceutical utopia….
This is a must read series you have so eloquently presented on this blog. Great work, and I thank you once again….
The use of powerful antipsychotic drugs has increased in children as young as three years old. Weight gain, increases in triglyceride levels and associated risks for diabetes and cardiovascular disease.
The average weight gain (adults) over the 12 week study period was the highest for Zyprexa—17 pounds. You’d be hard pressed to gain that kind of weight sport-eating your way through the holidays.
One in 145 adults died in clinical trials of those taking the antipsychotic drugs Zyprexa. This is Lilly’s # 1 product over $ 4 billion year sales,moreover Lilly also make billions on drugs that treat the diabetes often that has been caused by the zyprexa!
—
Daniel Haszard Zyprexa victim activist and patient who got diabetes from it. http://www.zyprexa-victims.com