
Eli Lilly ‘Ghostwrote’ Articles to Market Zyprexa, Files Show
Bloomberg
By Elizabeth Lopatto, Jef Feeley and Margaret Cronin Fisk
June 12, 2009… In one instance, Lilly employees contacted the Journal of Clinical Epidemiology about delays of an article criticizing a previously published piece linking Zyprexa, as well as the class of atypical antipsychotics, to diabetes. After Suraja Roychowdhury, Lilly’s senior scientific communications coordinator, wrote to the journal in November 2002, its editor, Andre Knottnerus, replied in an e-mail that it was “a bit strange to be contacted via the Lilly product team. Dr. Buse and coauthors can contact us directly next time.” Knottnerus was referring to the manuscript’s lead author, John Buse, a former president of the American Diabetes Association. A copy of the Nov. 22, 2002, e-mail was included in the unsealed documents.
Patrizia Cavazzoni, a Lilly staffer who co-wrote the article, e-mailed Buse on Jan. 9, 2003, seeking permission to send a separate e-mail asking to expedite publication. She also asked Buse if he would prefer “to send it in your name?” It isn’t clear from the e-mail chain whether the e-mail was sent by Buse or Cavazzoni. On Jan. 22, 2003, Buse e-mailed Cavazzoni to say he hadn’t heard anything and to request Knottnerus’s telephone number, according to the documents.
The Zyprexa article by Buse and Cavazzoni was a review of another submission that had previously appeared in the journal, according to the documents. That article summarized previous medical literature on atypical antipsychotics, and found Zyprexa had an increased risk of causing diabetes relative to the class…
-
High blood sugar [hyperglycemia]. High blood sugar can happen if you have diabetes already or even if you have never had diabetes. In rare cases, this could lead to ketoacidosis (build up of acid in the blood due to ketones), coma, or death. Your doctor should do lab tests to check your blood sugar before you start taking ZYPREXA and during treatment. In people who do not have diabetes, sometimes high blood sugar goes away when ZYPREXA is stopped. People with diabetes and some people who did not have diabetes before taking ZYPREXA need to take medicine for high blood sugar even after they stop taking ZYPREXA. If you have diabetes, follow your doctor’s instructions about how often to check your blood sugar while taking ZYPREXA.
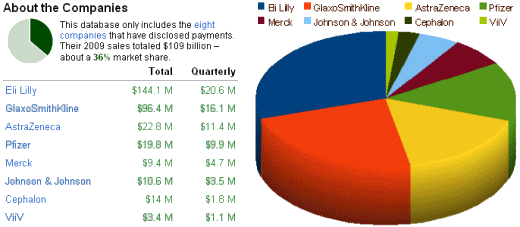
In fact, I’ve had enough of the Zyprexa story. It’s monotonous, boring. Turn over any rock and there’s another egregious example of their misbehavior. I read that they’re heading for the skids when Zyprexa‘s patent expires this year. Lilly says they’re determined to "stay independent" and not be acquired by some other pharmaceutical [Eli Lilly "very much opposed" to merger: CEO]. Who would want them with all of their accumulated liability? It’s like buying an asbestos manufacturer. But what do I know? Lilly is still on the top of the chart for paying doctors to be drug reps [speaker’s bureaus] in the ProPublica database:
Your post are well done I applaud your blog.I am now living with Zyprexa caused diabetes,given “off Label” 1996-2000 pre black warning label.
My first shock was an A1C of 14.6.-Daniel Haszard
I applaud the work too, thanks.
It took me 6 weeks of hard core intractable insomnia then I was free of Zyprexa.
Soooo,as an experienced patient I suggest to titrate, cut down in increments don’t go cold turkey.
Danniel Haszard http://www.zyprexa-victims.com