Perhaps Dr. Trivedi might consider that this piece at the end of his paper has something to do with the problem:
Competing interests
Madhukar H. Trivedi, M.D. has been a consultant for Abbott Laboratories, Inc.; Akzo (Organon Pharmaceuticals Inc.); AstraZeneca; Bayer; Bristol-Myers Squibb Company; Cephalon, Inc.; Cyberonics, Inc.; Eli Lilly & Company; Fabre-Kramer Pharmaceuticals, Inc. Forest Pharmaceuticals; GlaxoSmithKline; Janssen Pharmaceutica Products, LP; Johnson & Johnson PRD; Eli Lilly & Company; Meade Johnson; Neuronetics; Parke-Davis Pharmaceuticals, Inc.; Pfizer, Inc.; Pharmacia &; Upjohn; Sepracor; Solvay Pharmaceuticals, Inc.; VantagePoint; and Wyeth-Ayerst Laboratories.
He has served on speakers bureaus for Abdi Brahim; Akzo (Organon Pharmaceuticals Inc.); Bristol-Myers Squibb Company; Cephalon, Inc.; Cyberonics, Inc.; Forest Pharmaceuticals; GlaxoSmithKline; Janssen Pharmaceutica Products, LP; Eli Lilly & Company; Pharmacia &; Upjohn; Solvay Pharmaceuticals, Inc.; and Wyeth-Ayerst Laboratories.
He has also received grant support from Bristol-Myers Squibb Company; Cephalon, Inc.; Corcept Therapeutics, Inc.; Cyberonics, Inc.; Eli Lilly & Company; Forest Pharmaceuticals; GlaxoSmithKline; Janssen Pharmaceutica; Merck; National Institute of Mental Health; National Alliance for Research in Schizophrenia and Depression; Novartis; Pfizer Inc.; Pharmacia &; Upjohn; Predix Pharmaceuticals; Solvay Pharmaceuticals, Inc.; and Wyeth-Ayerst Laboratories.
Those papers by Dr. Trivedi of TMAP [
Texas Medication Algorithm Project] and his
Competing Interests sure must’ve struck a nerve, because since then I can’t seem to stop writing about the Pharmaceutical Industry’s invasion of Psychiatry and the misuse of the term
evidence-based medicine. I occupied myself in these last months catching up on the world of Clinical Trials and the Atypical Antipsychotics, and was as horrified by what I read as most anyone would be if they were really aware of what has gone on. It’s a pretty gruesome tale. And that lead me to the
STAR*D trial of the antidepressants. My reason for picking it was that it was an NIMH trial, and I wanted to see if the government funded trials were more reputable than the ones financed by PHARMA. In the course of looking at the Atypical Antipsychotics, I ran across CATIE [
Clinical Antipsychotic Trials in Intervention Effectiveness] and had been impressed that it was one that I trusted [for a change] and found myself going back to. So I thought I’d see how the NIMH did on Depression [not so hot is the short answer]:
In fact, the more I think about it, the more stirred up I get. It wasn’t lost on me that on every paper about
STAR*D, there was Dr. Madhukar H. Trivedi’s name – my old friend from those Algorithm papers, the man from TMAP [
Texas Medication Algorithm Project]. I didn’t think much about it until I read
STAR*D: What Have We Learned? by A. John Rush, M.D. [
American Journal of Psychiatry 164:201-204, February 2007]. It was a follow-up editorial by the first author of the
STAR*D report [along with Dr. Trivedi]. By the time I read Rush’s editorial, I’d been over
STAR*D enough times to realize that Dr. Rush was editorializing about what he wished that trial had said, not what it really said. And he seemed to be writing a sermon about Primary Care Physicians taking over the care of the mentally ill [
a slow learner…], so I did the sensible thing in our modern world, I
googled him. It turns out that he’s in Singapore as the CEO of something – it looks like it’s a Clinical Research Center for drug trials, affiliated with Duke [
a slow learner…]. Well. what had he been before that?
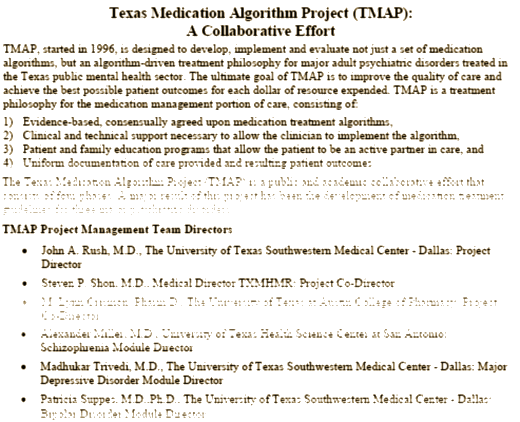
And there they were, Rush and Trivedi, TMAPers one and all. So I’m back where I started in January. What in the hell is TMAP? What new intrigue have I happened onto this time? said 1boringoldman who is suffering from the chronic intrigue syndrome.
Worse than you’d imagine. Here are some definitive references, then I’ll summarize a few high points as best I can [it’s a pretty convoluted story]…
-
-
-
Allen Jones, an investigator in the Pennsylvania inspector general’s office, began to look in to a program being started there for regulating the use of psychotropic medication in Medicaid and Medicare patients. It was modeled on the TMAP program in Texas. Here’s his description of TMAP:
TMAP began in 1995 as an alliance of individuals from within the pharmaceutical industry and the Texas state university, mental health and corrections systems. Start-up funds included a 1.7 million dollar grant from the Robert Wood Johnson Foundation; a Johnson&Johnson related foundation. Johnson&Johnson owns the pharmaceutical companies Janssen Pharmaceutica and Janssen/Ortho McNeil. The group’s goal was to develop a model mental health treatment program for incorporation into public mental health and prison systems. This model program would ensure that newer, expensive medications would be heavily used. But the drug industry had a problem: Clinical trials simply did not favor their new products. Alternative justification for favoring these drugs would have to be developed.
This consortium sought to “legitimize” the medications recommended in the model program’s “drug menus”. The group elected to utilize “Expert Consensus Guidelines”, rather than clinical studies or drug trials to form these recommendations. Essentially, TMAP opted to “establish” new drugs as the best drugs for various illnesses by surveying the opinions of doctors and psychiatrists of TMAP’s own choosing. No hard science, no patients, no study review, and no clinical trials – just the “Expert Opinions” of persons TMAP elected to survey.
The “Expert Consensus” process became TMAP’s standard mechanism for creating the appearance of superiority for certain drugs and it was employed repeatedly from 1996 to 2003. The doctors who were surveyed included persons who had already published articles favoring the new drugs. The survey included doctors with strong ties to the drug industry.[3. page 6]
Sound like a whistle-blower? He was. He started investigating, and was told to back off. He didn’t, so he got fired in Pennsylvania, but he kept on investigating as a private citizen.
"The protocol was developed with unrestricted educational grants from the following pharmaceutical companies: Abbott, Bristol-Myers Squibb, Eli Lilly, Forest, Glaxo-Wellcome, Janssen, Novartis, Pfizer, U.S. Pharmacopeia and Wyeth-Ayerst." [2.]
The TMAP protocols gave the treating Physicians a choice of drugs, all of them being the expensive proprietary ones:
"The drugs recommended in TMAP include Risperdal, Zyprexa, Seroquel, Geodon, Depakote, Paxil, Zoloft, Celexa, Wellbutrin, Zyban, Remeron, Serzone, Effexor, Buspar, Adderall, and Prozac, all manufactured by the above companies"… "The psychiatrists in charge of determining the TMAP guidelines all had major ties to the drug companies that funded its development, at least three of them — A. JOHN RUSH, ALEX MILLER and MADHUKAR TRIVEDI — owning stock options or having other financial business with them. Of the 46 members of the three panels, 27 had conducted research on behalf of pharmaceutical companies, served on drug company speakers’ bureaus or served as consultants to a drug company." [2.]
• Late 1990s-early 2000s: Texas mental health official Dr. Steven Shon travels around the country speaking about TMAP. Sixteen other states eventually adopt the protocol.
• 2003: The President’s New Freedom Commission on Mental Health — headed by Michael Hogan connections to drug companies that developed TMAP—recommends TMAP.
• 2004: Groups like NAMI publicly endorse TMAP. Daryl Regier, director of research at the APA lauded TMAP and called for increased funding of it. He is Executive Director of the APA’s “non-profit” research group APIRE (American Psychiatric Institute for Research and Education) Scholars in Research Program, which receives grants from Janssen and Eli Lilly, two of the drug companies that funded development of TMAP.
• 2004: After questioning drug company payments to state officials, whistleblower Allen Jones was fired from his job as an investigator at the Pennsylvania Inspector General’s office.
• 2004: Because the drug protocol used by many states originated in Texas, Jones filed a lawsuit in Travis County District Court against Johnson & Johnson and some subsidiaries. The lawsuit was sealed from public view because of protections that whistleblowers such as Jones are granted.
• October 2006: Shon was forced by superiors to retire from the Texas health department after officials learn of findings of a Texas Attorney General investigation into whether drug companies unduly influenced Shon.
• December 2006: Texas Attorney General joins Jones’ lawsuit. The lawsuit was opened to the public.
• August 2008: The Texas AG’s Office suspended a similar program tailored for children called CMAP, because of the allegations of drug companies influencing researchers.
And what does all of this have to do with John Rush, Director of TMAP, Principle Investigator of the NIMH funded STAR*D trial, and first author of the main STAR*D articles?
John Rush, the director of a controversial Texas program called T-MAP, which was created to implement a state system for treating psychiatric disorders, has taken a job in Singapore, where he has joined the Duke-NUS Graduate Medical School Singapore as vice dean for clinical sciences. [Look here].
Why is TMAP controversial? The state filed a lawsuit against Johnson & Johnson’s Jannsen unit for allegedly using false advertising and improper influence – such as grants, trips and other perks – to get its Risperdal antipsychotic on the now-mandatory adult protocol, the Texas Medication Algorithm Project. Drugmakers also reportedly paid decision makers to promote their meds. Just last month, Texas officials suspended a similar program tailored for children, called TC-MAP, over fears drugmakers may have given researchers consulting contracts, speaking fees or other payments to help get their products listed on the protocol.
“TMAP and TCMAP proved to be powerful marketing tools for Risperdal… Driven by these gains and revenues, defendants turned to developing a concerted marketing plan to replicate these programs, and the dramatic revenue and market share generated by TMAP and its progeny in other states,” the lawsuit states, according to The Daily Texan…
I don’t know if it shows, but this is a very hard story to run down, partly because it’s been veiled by the protections for whistle-blowers. But there are other things. The articles in Texas newspapers along the way are gone – not archived. The Texas Agency that TMAP was under has been reorganized and renamed, with no carry-over. I don’t even know if there’s still a TMAP. The suit is still going on, only recently released to move forward:
A Texas state court has ruled that a trial can proceed against Johnson & Johnson’s Janssen unit for allegedly using false advertising and improper influence – such as grants, trips and other perks – to ensure its Risperdal antipsychotic was placed on the mandatory protocol for the Texas Medication Algorithm Project, a state system protocol for treating psychiatric disorders.
The original lawsuit was filed in 2004 based on evidence provided by Allen Jones who, at the time, was working as a fraud investigator with the Pennsylvania Inspector General’s Office. Both Jones and the state of Texas are plaintiffs and their lawsuit contends Janssen engaged in a widespread scheme to ensure state Medicaid officials would give preferential treatment to Risperdal on TMAP [you can read the lawsuit here]. A lawyer who represents Jones says damages could exceed $1 billion, based on potential treble damages and penalties…

It feels like I was walking in the woods looking at the scenery and I stepped in a hole that turned out to be like the one in Lewis Carroll’s
Alice in Wonderland. I found myself in a place where everything sounds like
Jabberwoky. All I did was google John Rush to find out where he worked before he took his Singapore sojourn. I’ve left out huge pieces of this story – like the part about Governor George W. Bush’s enthusiasm for TMAP, or about how it bankrupted Texas Medicaid, or about President George W. Bush taking it to Washington as the NEW FREEDOM COMMISSION ON MENTAL HEALTH and TeenScreen, or a jillion other things that I ran across trying to make sense of it but didn’t even understand. Unlike little Alice in the illustration on the right, I can’t get my
vorpal blade to go
snicker-snack on this story. It’s too big.
But I know this for sure. It certainly has something to do with STAR*D and our attempts to understand its confusing presentation and conclusions. Like TMAP, somewhere in the last couple of years, STAR*D has gone underground. There have been a few papers about trivial side issues, but the main course remains locked in the NIMH kitchen, if there even is a main course. The timelines of the rise and fall of TMAP and STAR*D need to be drawn together, but that’s for another time. I need to take a shower and wash off the grime from this detour down the rabbit hole and into the tulgey wood…
 It feels like I was walking in the woods looking at the scenery and I stepped in a hole that turned out to be like the one in Lewis Carroll’s Alice in Wonderland. I found myself in a place where everything sounds like Jabberwoky. All I did was google John Rush to find out where he worked before he took his Singapore sojourn. I’ve left out huge pieces of this story – like the part about Governor George W. Bush’s enthusiasm for TMAP, or about how it bankrupted Texas Medicaid, or about President George W. Bush taking it to Washington as the NEW FREEDOM COMMISSION ON MENTAL HEALTH and TeenScreen, or a jillion other things that I ran across trying to make sense of it but didn’t even understand. Unlike little Alice in the illustration on the right, I can’t get my vorpal blade to go snicker-snack on this story. It’s too big.
It feels like I was walking in the woods looking at the scenery and I stepped in a hole that turned out to be like the one in Lewis Carroll’s Alice in Wonderland. I found myself in a place where everything sounds like Jabberwoky. All I did was google John Rush to find out where he worked before he took his Singapore sojourn. I’ve left out huge pieces of this story – like the part about Governor George W. Bush’s enthusiasm for TMAP, or about how it bankrupted Texas Medicaid, or about President George W. Bush taking it to Washington as the NEW FREEDOM COMMISSION ON MENTAL HEALTH and TeenScreen, or a jillion other things that I ran across trying to make sense of it but didn’t even understand. Unlike little Alice in the illustration on the right, I can’t get my vorpal blade to go snicker-snack on this story. It’s too big.
You are doing worthwhile stuff since you’ve stopped blogging about current events and politics. I admire your coping skills while the GOP tries to ram their so-called Christian values down our throats related to women issues like abortion. I never thought the news could get worse after Clinton or Bush left office but I was wrong. I’ve stopped watching the tv and I’m practically reading non stop fiction to distract me from the politics of the day. I wish the President would just stand before the cameras and say The GOP will not talk about jobs but what they can do to trash SS, medicare, clean air, and defunding womans and others healthcare and more. I hope to get them to look into their hearts and minds and help us help the people in this country who need jobs, safety in the workplace, shelter and food. Thank you for your time and leave the podium. What FOX news, the Koch brothers and the Supreme Court has done is so disheartning.
I miss keeping up too. The story of the Republican insanity continues. But right now, I’m drawn into the vortex of an equally appalling story that affects my own profession, a story that not so many people are trying to tell, so I’ve taken a turn in the road. I hope I’m doing it for a positive reason, focusing on a story that needs more voices rather than fleeing the garbled mess in Washington. There are just too many stories right now as we try to claw our way free of these
post-Bushpost-Reaganvery discouraging years…Mickey,
I started rolling on the floor laughing when I read, “chronic intrigue syndrome.” Now i know my diagnosis that i’d been missing for all of these years!
Thanks to you, I then quickly searched http://clinicaltrials.gov/ and found that there is a new trial just starting using Seroquel to treat this affliction so now you need to add that to your grid.
More seriously, great post. I knew about the apparent TMAP/Rush/Bush/STAR*D connection but your research and timeline puts it together more clearly.
I always viewed the grant of $1.2 million by the Agency for Health Care Research Quality to Rush & Trivedi to computerize their ‘measurement-based’ system of care as being a parting gift from the Bush administration since it was awarded in June 2008 despite this system having been such a colossal failure in STAR*D (see http://www.sciencedaily.com/releases/2008/06/080612070402.htm).
I must say though, since Trivedi got almost $5 million in 2010 funding from NIMH/NIDA there seems to be a truly bi-partisan effort to support shoddy research from Texas (see http://projectreporter.nih.gov/reporter_searchresults.cfm?&new=1&icde=7696821&loc=2&CFID=1637685&CFTOKEN=90911462).
It is very sad that these research dollars are being wasted in ways that only create mis-direction,
Ed
A few things that can help as you wade through all this are the overarching goals of all that activity:
1. Find more patients
2. Establish prescribing patterns
3. Not just who was involved but where did they come from
The New Freedom Commission and Teen Screen were both widely seen as trolling for patients, and not just any, but kids specifically. New Freedom was roundly criticized because at one point they were talking about wanting to screen all kids from infancy and start interventions to “prevent” mental illness. Teen Screen was widely criticized for using a Q&A with an extremely high false-positive rate and, initially at least, all kids in a school were given the test as an opt-out, not an opt in. BMJ covered all this in some detail a couple of years ago.
Establishing prescribing patterns–it wasn’t just that newer drugs were being pushed as in TMAP. STAR*D had everybody start on the same one–Citalopram. And level 2 included citalopram plus another one as half the treatment options. Pharma knows docs get comfortable with one drug, how it works, what the side effects are, etc. If you can get one drug to be seen as the agreed upon “starter” with others only considered if that one doesn’t work–that’s a marketer’s dream.
3. All these guys were from the same center in TX–and that’s where TMAP started. And GWB was the guy who led the parade on the New Freedom Commission.
“Find more patients” connects to the
Here’s some info on the New Freedom Commission and screening:
http://www.infowars.com/articles/brave_new_world/new_freedom_paul_amendment.htm
The school screening discussed there is a direct link to Teen Screen.
Sorry to keep going but you’re on to something. This is from testimony by an MD with kids, link below:
SF 2841 is proposed as part of the Roadmap for Mental Health System Transformation in Minnesota, which is an outgrowth of the federal New Freedom Commission report and the federal Mental Health Action Agenda. The Minnesota Roadmap clearly states what that plan is for young children. It proposes to, “…integrate early childhood screening systems to assure that all children ages birth to five are screened early and continuously for the presence of health, socioemotional or developmental needs†and then to implement, among other things, “mental health services and early care and education.â€
Members of the New Freedom Commission as well as groups advocating the Minnesota Roadmap plan have inherent financial, professional, and policy conflicts of interest and do not mention any scientific or medical problems with screening or treatment. For example, Michael Hogan, the chairman of the New Freedom Commission, was paid by the Janssen Pharmaceutica, the manufacturer of one of the drugs advocated in the model psychiatric drug treatment program (TMAP) in the commission’s report. The National Alliance for the Mentally Ill and the National Mental Health Association, both supporters, of this legislation and the Minnesota Roadmap received tax dollars from the federal mental health agency, SAMHSA, to help implement the New Freedom Commission’s recommendations, including universal screening and TMAP.
Even if mental health screening did not have these fatal policy and philosophical flaws, the medical and scientific justification for this idea is equally lacking. Proponents tell us that mental illnesses are biological brain disorders due to chemical imbalances of neurotransmitters, and that mental health screening is therefore scientific and objective and fully equivalent to hearing or blood pressure screening. They also tell us that children who screen positive will merely be sent for further evaluation, that screening does not yield a diagnosis, and that services do not necessarily mean drugs. Here is but a small sample of facts and statements from experts and the medical literature that contradict that view:
http://www.crossroad.to/articles2/006/edwatch/3-10-preschool-screening.htm
The TMAP procedural manuals used to be archived on the Texas Department of State Health Services (DSHS) Web site. A search of the site today produced no TMAP materials. Why would this historical record be removed from DSHS’s Web site?
Nancy,
Good question. I couldn’t find them anywhere…
http://www.utsouthwestern.edu/utsw/cda/dept353744/files/637891.html
Dual medications for depression increases costs,
side effects with no benefit to patients
Trivedi in the news again and in Dollars for docs database $$$ Pfizer
http://projects.propublica.org/docdollars/search?term=+Madhukar+H.+Trivedi&state%5Bid%5D=
The study was funded by the National Institute of Mental Health. Forest Pharmaceuticals, GlaxoSmithKline, Organon and Wyeth Pharmaceuticals provided the medications.
“Clinicians should not rush to prescribe combinations of antidepressant medications as first-line treatment for patients with major depressive disorder,†said Dr. Madhukar H. Trivedi, professor of psychiatry and chief of the division of mood disorders at UT Southwestern and principal investigator of the study, which is available online today and is scheduled for publication in an upcoming issue of the American Journal of Psychiatry.