-
Escitalopram [Lexapro] Plus Placebo (N=224)
-
Sustained-Release Bupropion [Wellbutrin SR] Plus Escitalopram [Lexapro] (N=221)
-
Extended-Release Venlafaxine [Effexor XR] Plus Mirtazapine [Remeron] (N=220)
Combining Medications to Enhance Depression Outcomes (CO-MED): Acute and Long-Term Outcomes of a Single-Blind Randomized Study
American Journal of Psychiatry
by A. John Rush, M.D., Madhukar H. Trivedi, M.D., Jonathan W. Stewart, M.D., Andrew A. Nierenberg, M.D., Maurizio Fava, M.D., Benji T. Kurian, M.D., Diane Warden, Ph.D., David W. Morris, Ph.D., James F. Luther, M.A., Mustafa M. Husain, M.D., Ian A. Cook, M.D., Richard C. Shelton, M.D., Ira M. Lesser, M.D., Susan G. Kornstein, M.D., and Stephen R. Wisniewski, Ph.D.
May 2, 2011
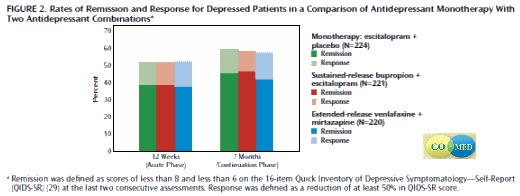
Objective: Two antidepressant medication combinations were compared with selective serotonin reuptake inhibitor monotherapy to determine whether either combination produced a higher remission rate in first-step acute-phase (12 weeks) and long-term (7 months) treatment.
Method: The single-blind, prospective, randomized trial enrolled 665 outpatients at six primary and nine psychiatric care sites. Participants had at least moderately severe nonpsychotic chronic and/or recurrent major depressive disorder. Escitalopram (up to 20 mg/day) plus placebo, sustained-release bupropion (upto 400 mg/day) plus escitalopram (up to 20 mg/day), or extended-release venlafaxine (up to 300 mg/day) plus mirtazapine (up to 45 mg/day) was delivered (1:1:1 ratio) by using measurement-based care.The primary outcome was remission, defined as ratings of less than 8 and less than 6 on the last two consecutive applications of the 16-item Quick Inventory of Depressive Symptomatology-Self-Report. Secondary outcomes included side effect burden, adverse events, quality of life, functioning, and attrition.
Results: Remission and response rates and most secondary outcomes were not different among treatment groups at 12 weeks. The remission rates were 38.8% for escitalopram-placebo, 38.9% for bupropion-escitalopram, and 37.7% for venlafaxine-mirtazapine, and the response rates were 51.6%–52.4%. The mean number of worsening adverse events was higher for venlafaxine-mirtazapine (5.7) than for escitalopram-placebo (4.7). At 7 months, remission rates (41.8%–46.6%), response rates (57.4%–59.4%), and most secondary outcomes were not significantly different.
Conclusions: Neither medication combination outperformed monotherapy. The combination of extended-release venlafaxine plus mirtazapine may have a greater risk of adverse events.
My other comment has to do with this whole line of thinking. STAR*D, CO-MED, and the coming study, iSPOT, are based on the premise that there’s some way to get more mileage out of the antidepressants or that there is some differential in response so groups of individuals might respond differently to different classes of antidepressants – that by tweaking some algorithm or testing some gene or another, the power of these medications will be enhanced. If that’s the case, so far the record is fairly clear – throwing money at the likes of John Rush, Madhukar Trivedi, Maurizio Fava, etc. is not going to be the way to answer the question. Neither STAR*D nor CO-MED has added much to the medical record – either way – other than fog and the costs are impressive.
Or that treatment for psychiatric disorders is entirely non-specific. Which is not good news for Big Pharma. Or Psychiatry as a whole.
Let’s see if I have this straight. STAR*D and CO-MED were two enormous, extremely well-funded studies of antidepressant effectiveness. (I’ll ignore the investigators’ shenanigans for now.) Both of them gave equivocal results, showing (a) no particular drug or drug combo does better than any other, and (b) drug treatment in general is fairly ineffective.
There are only three possible explanations for this: (1) The drugs DO work, we’re just not using them right (and thus we must await the era of personalized medicine); (2) The drugs are simply expensive placebos and do nothing to the biology of depression; or (3) we’ve been wrong about “depression” all along.
If (2) or (3) are correct, then I agree with Tom. Psychiatry as we know it will soon cease to exist.
Psychiatrists had plenty to do before there were antidepressants, before Prozac. The notion that the fate of Psychiatry hinges on the neuroscience of a particular class of drugs is a relatively recent idea, created by a small group of people – not our best or brightest. Ironically, they’re also the people who are writing books or running studies that attempt to transfer the treatment of mentally ill people to primary care physicians. Ultimately, both ideas – Psychiatry is equated with drug treatment and drug treatment can be done by primary care physicians – arose in the marketing departments of Pharma.
Thanks for the heads up on Co-Med. My question is who’s minding the ship at NIMH to allow the QIDS-Self Report to be used as the primary outcome measure in such a costly tax-payer funded study since the QIDS’s copyright holder is Rush the PI? That’s a rather significant conflict of interest.
I did a quick scan of the disclosure statements and noted that none were listed for Drs. Stewart or Nierenberg and several others. This is an oversight since both Stewart and Nierenberg disclosure conflicts in the April 2011 Journal of Clinical Psychopharmacology article, “Residual Symptoms in Depressed Outpatients Who Respond by 50% But Do Not Remit to Antidepressant Medication.â€
Here is Nierenberg’s listing in the JCP article. As you will see it is quite extensive. Perhaps ‘journal space’ issues prevent ALL authors from listing ALL conflicts. From JCP:
Dr Nierenberg has consulted to or served on the advisory boards of Abbott, Appliance Computing, Brain Cells, Bristol-Myers Squibb, Eli Lilly, EpiQ, Forest, GlaxoSmithKline, Janssen, Jazz,Merck, Novartis, Pamlab, Pfizer, PGx Health, Pharmaceutica, Schering-Plough, Sepracor, Shire, Somerset, Takeda, and Targacept; has received research support from Cederroth, Cyberonics, Forest, Medtronics, the National Alliance for Research on Schizophrenia and Depression, the National Institute of Mental Health, Ortho-McNeil-Janssen, Pamlab, Pfizer, Shire, and the Stanley Foundation through the Broad Institute; has received past support from Bristol-Myers Squibb, Cederroth, Eli Lilly, Forest, GlaxoSmithKline, Janssen, Lictwer Pharma, Pfizer, and Wyeth; has received honoraria from the Massachusetts General Hospital (MGH) Psychiatry Academy (MGHPA activities are supported through Independent Medical Education grants from AstraZeneca, Eli Lilly, and Janssen); earns fees for editorial functions for CNS Spectrums through MBL Communications, and Psychiatric Annals through Slack; receives honoraria as a CME executive director for the Journal of Clinical Psychiatry through Physicians Postgraduate Press; has been on the speaker’s bureaus of Bristol-Myers Squibb, Cyberonics, Eli Lilly, Forest, GlaxoSmithKline, and Wyeth; has received royalties from Cambridge University Press and Belvoir Publishing; owns stock options in Appliance Computing; and owns the copyrights to the Clinical Positive Affect Scale and the MGH Structured Clinical Interview for the Montgomery Asberg Depression Scale, exclusively licensed to the MGH Clinical Trials Network and Institute.
I couldn’t determine what they declared as their Primary Outcome instrument. In clinicaltrials.gov, it’s vague…
So sorry to waste space, but I decided to copy here (for shock value) the disclosures list on the CO-MED study:
Dr. Rush has received consulting fees from Advanced Neuromodulation Systems, Best Practice Project Management, Brain Resource, Otsuka, and the University of Michigan; he has received consultant/speaker fees from Forest; he has received consultant fees from and owns stock in Pfizer; he has received author royalties from Guilford Publications, Healthcare Technology Systems, and the University of Texas Southwestern Medical Center; and he has received research support from the National Institute of Mental Health. Dr. Trivedi has received research support from the Agency for Healthcare Research and Quality, Corcept, Cyberonics, Merck, NARSAD, the National Institute of Mental Health, the National Institute on Drug Abuse, Novartis, Pharmacia & Upjohn, Predix (EPIX), Solvay, and Targacept; he has received consulting and speaker fees from Abbott, Abdi Ibrahim, Akzo (Organon), AstraZeneca, Bristol-Myers Squibb, Cephalon, Eli Lilly, Evotec, Fabre Kramer, Forest, GlaxoSmithKline, Janssen, Johnson & Johnson, Meade Johnson, Medtronic, Neuronetics, Otsuka, Parke-Davis, Pfizer, Sepracor, Shire Development, VantagePoint, and Wyeth. Dr. Fava has received research support from Abbott, Alkermes, Aspect Medical Systems, AstraZeneca, BioResearch, BrainCells, Bristol-Myers Squibb, CeNeRx BioPharma, Cephalon, Clinical Trials Solutions, Clintara, Covidien, Eli Lilly, EnVivo, Euthymics Bioscience, Forest, Ganeden Biotech, GlaxoSmithKline, Icon Clinical Research, i3 Innovus/Ingenix, Johnson & Johnson, Lichtwer, Lorex, NARSAD, the National Center for Complementary and Alternative Medicine, the National Institute on Drug Abuse, NIMH, Novartis, Organon, Pamlab, Pfizer, Pharmavite, Photothera, RCT Logic, Roche, Sanofi-Aventis, Shire, Solvay, Synthelabo, and Wyeth; he has served as adviser or consultant to Abbott, Affectis, Alkermes, Amarin, Aspect Medical Systems, AstraZeneca, Auspex, Bayer, Best Practice Project Management, BioMarin, Biovail, BrainCells, Bristol-Myers Squibb, CeNeRx BioPharma, Cephalon, Clinical Trials Solutions, CNS Response, Compellis, Cypress, DiagnoSearch Life Sciences, Dinippon Sumitomo, DOV, Edgemont, Eisai, Eli Lilly, ePharmaSolutions, EPIX, Euthymics Bioscience, Fabre-Kramer, Forest, GenOmind, GlaxoSmithKline, Grunenthal, i3 Innovus/Ingenix, Janssen, Jazz, Johnson & Johnson, Knoll, Labopharm, Lorex, Lundbeck, MedAvante, Merck, MSI Methylation Sciences, Naurex, Neuronetics, NextWave, Novartis, Nutrition 21, Orexigen, Organon, Otsuka, Pamlab, Pfizer, PharmaStar, Pharmavite, PharmoRx, Precision Human Biolaboratory, Prexa, PsychoGenics, Psylin Neurosciences, Puretech Ventures, RCT Logic, Rexahn, Ridge Diagnostics, Roche, Sanofi-Aventis, Schering-Plough, Sepracor, Servier, Solvay, Somaxon, Somerset, Sunovion, Synthelabo, Takeda, Tal Medical, Tetragenex, Transcept, TransForm, and Vanda; he has received speaking or publishing fees from Adamed, Advanced Meeting Partners, the American Psychiatric Association, the American Society of Clinical Psychopharmacology, AstraZeneca, Belvoir Media Group, Boehringer Ingelheim, Bristol-Myers Squibb, Cephalon, CME Institute/Physicians Postgraduate Press, Eli Lilly, Forest, GlaxoSmithKline, Imedex, MGH Psychiatry Academy/ Primedia, MGH Psychiatry Academy/Reed Elsevier, Novartis, Organon, Pfizer, PharmaStar, United BioSource, and Wyeth; he owns stock in Compellis; he has a patent for SPCD, a patent application for a combination of azapirones and bupropion in major depressive disorder, and a patent for research and licensing of SPCD with RCT Logic and Lippincott, Williams & Wilkins; and he receives copyright royalties for the MGH CPFQ, SFI, ATRQ, DESS, and SAFER. Dr. Kurian has received research support from Evotec, Pfizer, and Targacept. Dr. Warden has owned stock in Bristol-Myers Squibb and Pfizer in the past 5 years. Dr. Husain has received research support from Cyberonics, Magstim, Neuronetics, NIH/NIMH, the Stanley Foundation, and St. Jude Medical; he has also served on advisory boards for AstraZeneca, BMS, Forest, and Novartis. Dr. Cook has served as an adviser and consultant for Ascend Media, Bristol-Myers Squibb, Cyberonics, Janssen, NeuroSigma, and the U.S. Departments of Defense and of Justice; he has served on the speakers bureaus for Bristol-Myers Squibb, Neuronetics, and Wyeth/Pfizer; he has received research support from Aspect Medical Systems/Covidien, Cyberonics, Eli Lilly, Neuronetics, NIH, Novartis, Pfizer, and Sepracor; his patents on biomedical devices are assigned to the Regents of the University of California. Dr. Shelton has received grant/research support from Abbott, AstraZeneca, Eli Lilly, GlaxoSmithKline, Janssen, Pamlab, Pfizer, Sanofi, and Wyeth; he has been a paid consultant to Evotec, Janssen, and Sierra Neuropharmaceuticals; and he has served on speakers bureaus for Abbott, Bristol-Myers Squibb, Eli Lilly, GlaxoSmithKline, Janssen, and Wyeth. Dr. Lesser has received research support from Aspect Medical Systems and NIMH. Dr. Kornstein has received grants/research support from Boehringer-Ingelheim, Bristol-Myers Squibb, Eli Lilly, Forest, the National Institute of Mental Health, Novartis, Pfizer, Rexahn, Takeda, and Wyeth; she has served on advisory boards for Bristol-Myers Squibb, Dey, Eli Lilly, Forest, Pfizer, PGx Health, Takeda, and Weth; and she has received book royalties from Guilford Press. Dr. isniewski reports financial relationships with Cyberonics (2005– 009), ImaRx Therapeutics (2006 ), Bristol-Myers Squibb (2007–2008 ), Oanon (2007), Case-Western University (2007), Singapore Clinical Rsearch Institute (2009), Dey (2010), and Venebio (2010).
Do these people have any time to see patients? (Serious question.)
No waste. I was just posting on that same list. I find it beyond shameful. There ought to be a law, really!
Oops! Sorry I missed your link to the COI listing. Kudos for the much cleaner format.
I don’t think any conclusions can be drawn from this flawed study: http://treatmentasusual.wordpress.com/2011/07/22/co-med-trial-results-are-in-blame-the-patients/