New York Times
By MELODY PETERSEN
February 01, 2004
The drug industry has created vast markets for products like Viagra, Celebrex and Vioxx by spending billions of dollars on consumer advertising. But to sell medicines that treat schizophrenia, the companies focus on a much smaller group of customers: state officials who oversee treatment for many people with serious mental illness. Those patients – in mental hospitals, at mental health clinics and on Medicaid – make states among the largest buyers of antipsychotic drugs. For Big Pharma, success in the halls of government has required a different set of marketing tactics. Since the mid-1990’s, a group of drug companies, led by Johnson & Johnson, has campaigned to convince state officials that a new generation of drugs – with names like Risperdal, Zyprexa and Seroquel – is superior to older and much cheaper antipsychotics like Haldol…
TMAP began in 1995 as an alliance of individuals from within the pharmaceutical industry and the Texas state university, mental health and corrections systems. Start-up funds included a 1.7 million dollar grant from the Robert Wood Johnson Foundation; a Johnson&Johnson related foundation. Johnson&Johnson owns the pharmaceutical companies Janssen Pharmaceutica and Janssen/Ortho McNeil…The group’s goal was to develop a model mental health treatment program for incorporation into public mental health and prison systems. This model program would ensure that newer, expensive medications would be heavily used. But the drug industry had a problem: Clinical trials simply did not favor their new products. Alternative justification for favoring these drugs would have to be developed.
This consortium sought to “legitimize” the medications recommended in the model program’s “drug menus”. The group elected to utilize “Expert Consensus Guidelines”, rather than clinical studies or drug trials to form these recommendations. Essentially, TMAP opted to “establish” new drugs as the best drugs for various illnesses by surveying the opinions of doctors and psychiatrists of TMAP’s own choosing. No hard science, no patients, no study review, and no clinical trials – just the “Expert Opinions” of persons TMAP elected to survey…
The Texas Medication Algorithm Project: Development and Implementation of the Schizophrenia Algorithm
by John A. Chiles, M.D., Alexander L. Miller, M.D., M. Lynn Crismon, Pharm.D., A. John Rush, M.D., Amy S. Krasnoff, M.A. and Steven S. Shon, M.D.
Psychiatric Services 50:69-74, January 1999… For several reasons, the initial algorithm for schizophrenia was limited to pharmacotherapy. First, the literature on medications provides significant evidence on which to base clinical consensus. Second, although medications constitute a small portion of the mental health budget, the increased costs of new medications have caused administrative scrutiny and, in many cases, have resulted in limitations on physicians’ prescribing choices. Third, appropriate pharmacotherapy may decrease utilization of crisis services and increase the demand for psychosocial rehabilitation programs. Data from implementation of the medication algorithm might inform the development of uniformly applied, consensus-driven, psychosocial rehabilitation programs. The basis in the literature for the algorithm was determined by a multistep process. First, the codirectors for development of the schizophrenia algorithm [the first and second authors] made explicit the "facts" about schizophrenia in which they had a great degree of confidence…
It sounds so damned good it almost makes you wish it were true – like fighting Nazis, or the Red Menace of World Communism. If you read through those "facts," they lead to only one conclusion – use Atypical Antipsychotics. But the facts weren’t true. And the drug companies knew they weren’t true. They’d tried to prove these things in their clinical trials, but they couldn’t make them work. Here’s a reminder or two from my previous posts on Seroquel and Zyprexa:
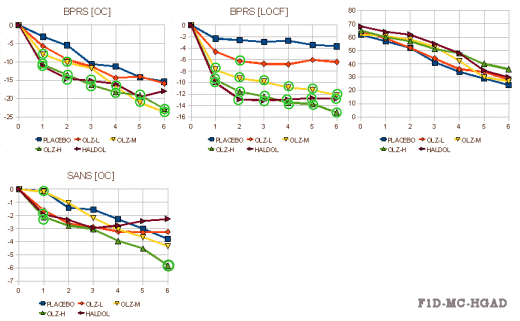
Trial HGAD
This trial compared Placebo vs Olanzapine in three dose ranges vs Haldol. The results [from the FDA Review tables][the right graph is the drop-outs]:
Olanzapine versus placebo and haloperidol: acute phase results of the North American double-blind olanzapine trial
by Beasley CM Jr, Tollefson G, Tran P, Satterlee W, Sanger T, Hamilton S., and the Olanzapine Study Group
Neuropsychopharmacology. 1996 Feb;14(2):111-23.
From the Psychopharmacology Division, Lilly Research Laboratories, Eli Lilly and Company. Acknowledgments: The authors thank Nancy C. Andresen,MD, PhD, Andrew H. Woods Professor of Psychiatry, University of Iowa College of Medicine, Iowa City, IA, for assistance in planning the study, providing rating scale training, and serving as principal investigator; Jan Hanshew, Marcia Nordman, Mindy Davis, MTSC, Mary Ann Heath, Carol Mattler, Pat Cowell-Hanover, PhD, Marie Reamer, Ray Albritton, Tom Voegele, and Charlotte Luttman for assistance in study administration and manuscript preparation.

Trial HGAJ
Olanzapine versus haloperidol in the treatment of schizophrenia and schizoaffective and schizophreniform disorders: results of an international collaborative trial
by Tollefson GD, Beasley CM Jr, Tran PV, Street JS, Krueger JA, Tamura RN, Graffeo KA, Thieme ME.
American Journal of Psychiatry. 1997 Apr;154(4):457-65.From Lilly Research Laboratories. Address reprint requests to Dr. Tollefson, Lilly Research Laboratories, Eli Lilly and Company, Lilly Corporate Center, Drop Code 0538, Indianapolis, IN 46285. The authors thank Suzanne Myers for technical and editorial assistance in the preparation of this manuscript.
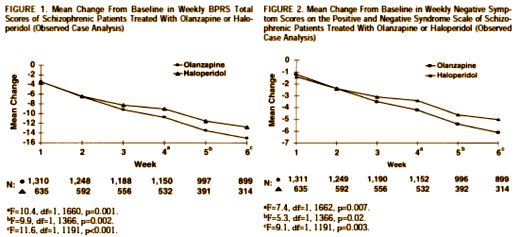
Objective: This international, multicenter double-blind trial was designed to compare the therapeutic profile of an atypical antipsychotic, olanzapine, with that of a conventional dopamine D2 antagonist, haloperidol.Method: A total of 1,996 patients at 174 sites in Europe and North America were randomly assigned to treatment with olanzapine (N=1,336) or haloperidol (N=660) over 6 weeks. The primary efficacy analysis involved the mean change from baseline to endpoint in total scores on the Brief Psychiatric Rating Scale (BPRS). Secondary analyses included comparisons of the mean change in positive and negative symptoms, comorbid depression, extrapyramidal symptoms, and overall drug safety.Results: Olanzapine demonstrated clinical results superior to those of haloperidol on overall improvement according to the BPRS and on every secondary measure, including depression. Olanzapine was also associated with significantly fewer discontinuations of treatment due to lack of drug efficacy or adverse events. Substantially more olanzapine-treated patients (66.5%) than haloperidol treated patients (46.8%) completed 6 weeks of therapy. Statistically significant advantages of olanzapine treatment were related to 1) change in negative symptoms, 2) extrapyramidal symptom profile, 3) effect on prolactin levels, and 4) response rate.Conclusions: Olanzapine shows a superior and broader spectrum of efficacy in the treatment of schizophrenic psychopathology, with a substantially more favorable safety profile, than haloperidol. It meets several of the criteria for a novel atypical antipsychotic agent.

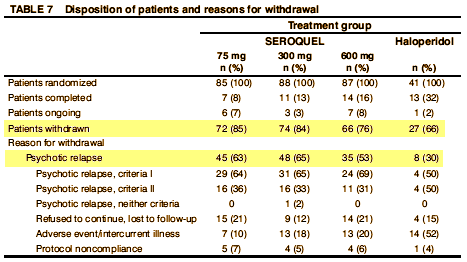
Then there was Seroquel and their famous pre-approval Study 15 – never published. But Trial 0015 is avaliable now for all to see. Here’s one of the internal documents about it:

There was no statistically significant dose response among SEROQUEL groups in the time to withdrawal from the trial in the intent to treat population, which was the primary efficacy variable. Pairwise comparison showed no statistically significant differences among any treatment group, including haloperidol, in the time to withdrawal from the trial. There was also no statistically significant dose.response among the SEROQUEL groups in the time to withdrawal from the trial for psychotic relapse. Times to psychotic relapse were generally longer in the haloperidol group. Pairwise comparisons of the time to withdrawal for psychotic relapse revealed statistically significant differences between the haloperidol group and each SEROQUEL group. However, there was an imbalance in the reasons for withdrawal from the trial between the SEROQUEL and haloperidol treatment groups with proportionally more patients in the haloperidol group withdrawing for adverse events. Since proportionally more censoring for relapse occurred in the haloperidol treatment group, any contrasts between the haloperidol and SEROQUEL groups for time to withdrawal for psychotic relapse may not reflect true differences in the relapse distributions among the groups and therefore are non.informative. Results of the analysis of the time to withdrawal in the secondary population showed a similar trend as seen in the intent to treat population. The analysis of prognostic variables indicated that only the interaction between treatment groups and the need for neuroleptic medication to be tapered during Segment A were significantly associated with time to withdrawal.
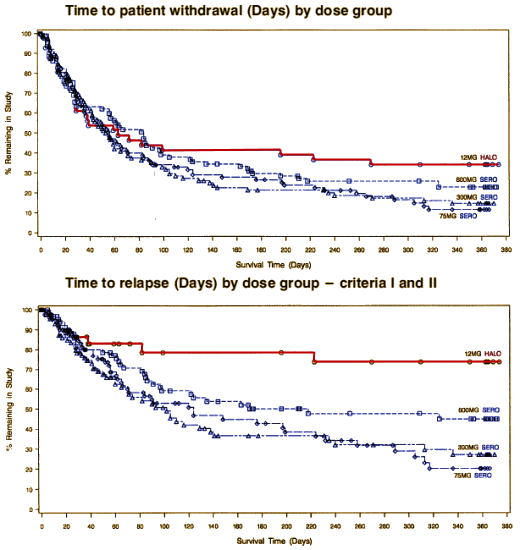
Well, I think it’s relevant to efficacy. What it shows is that Seroquel is way less efficacious than Haldol [in spite of Haldol’s side effects].
The "facts" weren’t close to true as it turned out, but they were a venus fly trap catching flies. In my last post, I showed the 1999 TMAP Algorithm for Schizophrenia. By then, Seroquel was on the market and made the cut.
But it wasn’t out yet in 1996 with their original algorithm:
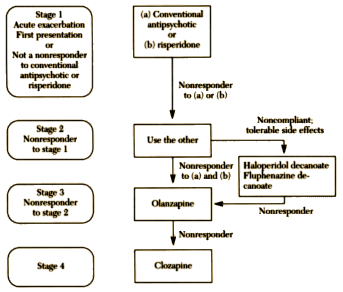

This is one of those posts so jam-packed w great info and commentary, it’s fantastic. thanks, so glad to see someone dig into TMAP.