They submitted two identical studies: USA-79 and INT-6. They were both Active Comparator Trials [Risperdal versus Haldol]. In USA-79, Risperdal won in both the time to relapse race [primary outcome] and in the PANSS scores, significantly beating Haldol in both positive and negative symptom reduction [secondary outcome]:
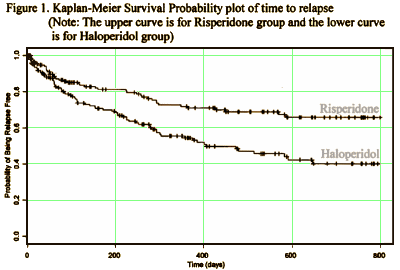
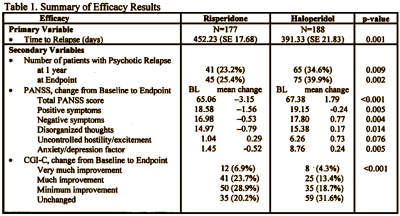
In INT-6, there was no significant statistical difference between Risperdal and Haldol in anything. That was interpreted as evidence of efficacy, but not of superiority to Haldol:

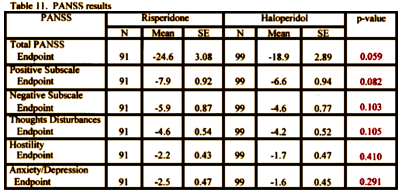
reformatted to match USA-79
Adverse events were pooled for the two studies:
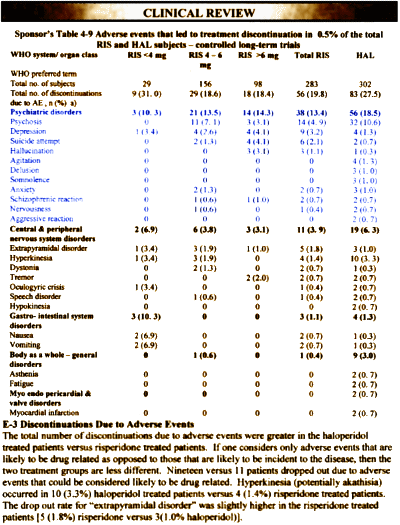
As they say, the adverse events due to drug effects are similar [EPSris>EPShal].
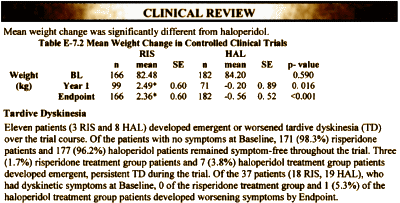
So when it’s all said and done, in the initial Risperdal studies available at the time, Risperdal only really fit one of the "facts" – "…reduces the likelihood of tardive dyskinesia". At that point, the weight gain and diabetes potential was not publicly apparent.
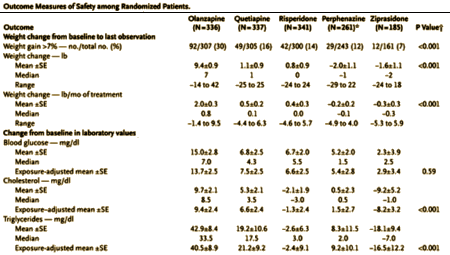
We, of course, knew a lot more later. With CATIE [Clinical Antipsychotic Trials in Intervention Effectiveness][an N.I.M.H. funded study][2005] and the European schizophrenia outpatient health outcomes study [financed by Eli Lilly][2010], the efficacy comparisons were finally solidified:
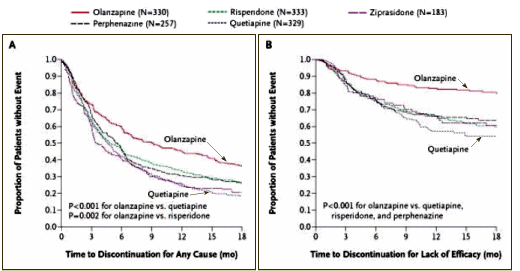
CATIE
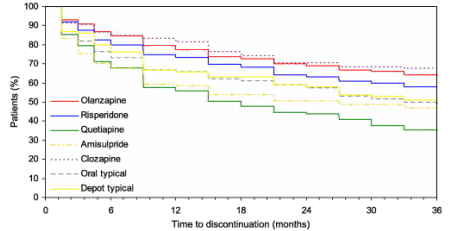
EUR SCHIZ
And as for EPS and TD:
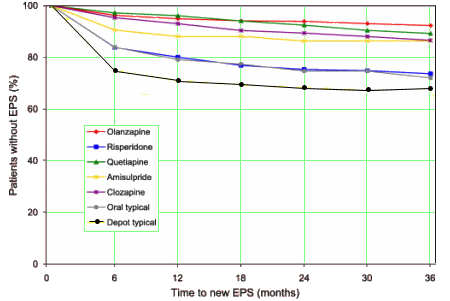
EUR SCHIZ
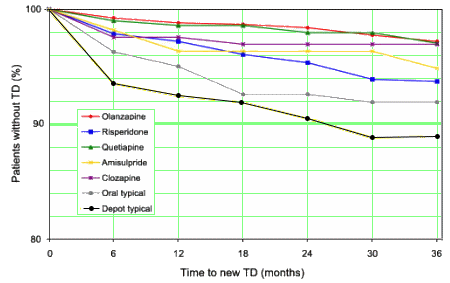
EUR SCHIZ
Consensus Development Conference on Antipsychotic Drugs and Obesity and Diabetes
American Diabetes Association, American Psychiatric Association, American Association of Clinical Endocrinologists, and North American Association for the Study of Obesity
Diabetes Care 27:596–601, 2004.The SGAs are of great benefit to a wide variety of people with psychiatric disorders. As with all drugs, SGAs are associated with undesirable side effects. One constellation of adverse effects is an increased risk for obesity, diabetes, and dyslipidemia. The etiology of the increased risk for metabolic abnormalities is uncertain, but their prevalence seems correlated to an increase in body weight often seen in patients taking an SGA. Direct drug effects on β-cell function and insulin action could also be involved, since there is insufficient information to rule out this possibility. In the general population, being overweight or obese also carries a much higher risk of diabetes and dyslipidemia. These three adverse conditions are closely linked, and their prevalence appears to differ depending on the SGA used. Clozapine and olanzapine are associated with the greatest weight gain and highest occurrence of diabetes and dyslipidemia. Risperidone and quetiapine appear to have intermediate effects. Aripiprozole and ziprasidone are associated with little or no significant weight gain, diabetes, or dyslipidemia, although they have not been used as extensively as the other agents.
Drug Weight Diabetes Lipids Clozapine +++ + + Olanzapine +++ + + Risperidone ++ D D Quetiapine ++ D D Aripiprazole * +/− − − Ziprasidone * +/− − − + = increase effect; − = no effect; D = discrepant results
* = Newer drugs with limited long-term data.The choice of SGA for a specific patient depends on many factors. The likelihood of developing severe metabolic disease should also be an important consideration. When prescribing an SGA, a commitment to baseline screening and follow-up monitoring is essential in order to mitigate the likelihood of developing CVD, diabetes, or other diabetes complications.

Sorry, the comment form is closed at this time.