The judgement in the South Carolina Risperdal case yesterday focuses our attention on the marketing practices of pharmaceutical companies in the last decade when the Atypical Antipsychotics were introduced. Back then there was a lot of excitement about these new drugs. The first generation neuroleptics were both ‘wonder drugs’ because of their effect on psychotic symptoms and big problems because of their side effects [EPS and Tardive Dyskinesia] and to a lesser extent because of what they didn’t do [negative symptoms]. The ‘wonder’ of finally being able to treat psychosis had worn off and we were left with the problem of homeless mental patients and the specter of permanent neurological damage from the treatment. The most effective drug, Clozapine, carried an ever more devastating side effect – fatal agranulocytosis. So when Clozapine derivatives like Risperdal, Zyprexa, and Seroquel, without that ominous side effect became available, there was a ready audience of patients, health care systems, and psychiatrists eager to use them. Had I been in a place at that point to treat Schizophrenic patients, I would have been excited too. Anyone would’ve been.
The other force is what the pharmaceutical companies call the life cycle of a drug. The contract that’s evolved over the years makes good sense and no sense at all at the same time. It essentially says that because you developed the drug, we’ll give you x years of exclusive rights to make and sell your drug and we’ll tolerate your big profits to pay you back for your efforts, but then it’s over and the generic version will be available to all cheaply. It makes sense because it promotes the development of new treatments. It makes no sense because they hit the ground running and overlook if possible problems that come up. The drug companies try to get as many indications as possible and overplay the benefits and downplay the problems in order to get maximum returns on their investment during their moment on the stage. The Atypical Antipsychotics arrived in the peak of the age of psychopharmacology, following on the heels of the success of the selective serotonin reuptake inhibitors. They were the new "blockbusters" on the scene.
What we wanted was the antipsychotic potency of Clozapine, and no [or at least less] neurologic side effects [particularly irreversible ones]. It would also be nice to have no new problems. That’s not what these drugs had to offer to everyone’s disappointment. Some of these suits have to do with the allegation that the drug companies specifically tried to keep us in the dark about their drug’s problems, even though they knew about them. The suits in Louisiana, West Virginia, and South Carolina against J&J’s Risperdal are in that category. A pivotal episode in the cases had to do with a "Dear Doctor" letter. The FDA had concluded that all of these drugs had the possibility of causing or unearthing diabetes – no small problem. At the end of 2003, they required the companies add a warning and notify doctors. J&J responded with a "Dear Doctor" letter [in purple] that was, if anything, an advertisement:

This example [and others] is being seen as knowingly using false advertising. J&J lost the suit in Louisiana and is appealing. They lost in West Virginia, but were successful on appeal. J&J vows to appeal this South Carolina ruling from yesterday. The suit in Pennsylvania that got thrown out of court was different. The false advertising part was in there, but it also had to do with something else – tricking the State into purchasing Risperdal for its charity and State funded programs.
 The suit in Texas that’s now scheduled for November is a central case in this series. It originated in Pennsylvania when Penn OIG inspector Allen Jones found evidence that the drug companies were funding government officials directly to influence selecting their drugs. Frustrated that no one was acting on his findings, he went to the press and was fired. Jones filed a whistle-blower suit in Texas, where that State’s TMAP program had become implicated as the central instrument for exerting influence on medication purchases in Texas and many other States – worth billions to the drug companies. It’s a much bigger allegation than false advertising. The list of charges is extensive [and damning]. Though it’s a civil suit, it reads like a criminal indictment. What the suit says is that the Texas Medical Algorithm Project was created with funding from Pharmaceutical Sponsors, and that its actual purpose was to induce the State to use the Sponsor’s products rather than the much less expensive generic alternatives. That is essentially a conspiracy to defraud the State of Texas and ultimately 15 other states:
The suit in Texas that’s now scheduled for November is a central case in this series. It originated in Pennsylvania when Penn OIG inspector Allen Jones found evidence that the drug companies were funding government officials directly to influence selecting their drugs. Frustrated that no one was acting on his findings, he went to the press and was fired. Jones filed a whistle-blower suit in Texas, where that State’s TMAP program had become implicated as the central instrument for exerting influence on medication purchases in Texas and many other States – worth billions to the drug companies. It’s a much bigger allegation than false advertising. The list of charges is extensive [and damning]. Though it’s a civil suit, it reads like a criminal indictment. What the suit says is that the Texas Medical Algorithm Project was created with funding from Pharmaceutical Sponsors, and that its actual purpose was to induce the State to use the Sponsor’s products rather than the much less expensive generic alternatives. That is essentially a conspiracy to defraud the State of Texas and ultimately 15 other states:
That certainly happened between TMAP1996 and TMAP1999.

And TMAP was industry funded:

I know nothing of the law or how such things play out in a courtroom, but this looks really rotten to me. It’s hard to imagine thinking that one could get away with such things, but 1996-1999 was a different time and they were a lot further off of the radar in those days. Likewise, notice that the NIMH was also a funding agency for TMAP. Recall also that the involved psychiatrists were faculty members of the Departments of Psychiatry in three University of Texas medical schools. One of the main authors of TMAP Schizophrenia Algorithm, John A. Chiles, retired from UT in 2000 after the Algorithm was changed [above]. He returned home to Washington State where he had a consulting business, involved in implementing TMAP in other places [paid by whom?].
The Texas Medication Algorithm Project: Clinical Results for Schizophrenia
by Alexander L. Miller, M. Lynn Crismon, A. John Rush, John Chiles, T. Michael Kashner, Marcia Toprac, Thomas Carmody, Melanie Biggs, Kathy ShoreS’Wilson, Judith Chiles, Brad Witte, Christine BoW’Thomas, Dawn I. Velligan, Madhukar Trivedi, Trisha Suppes, and Steven Short
Schizophrenia Bulletin, 30(3):627-647,2004.
reformatted for clarity
reformatted for clarity
An Empirical Analysis of Cost Outcomes of the Texas Medication Algorithm Project
by T. Michael Kashner, Ph.D., J.D., A. John Rush, M.D., M. Lynn Crismon, Pharm.D., Marcia Toprac, Ph.D., Thomas J. Carmody, Ph.D., Alexander L. Miller, M.D., Madhukar H. Trivedi, M.D., Annie Wicker, B.S. and Trisha Suppes, M.D., Ph.D.
Psychiatric Services 57:648-659, 2006.
reformatted for clarity
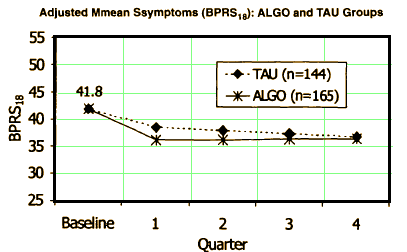
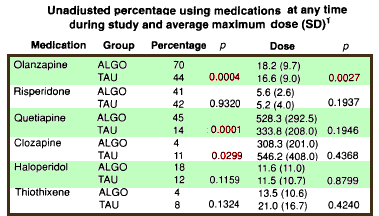
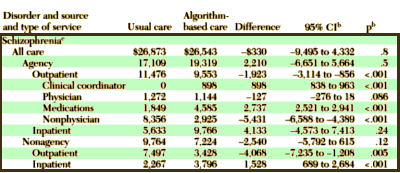
They reported that the two groups didn’t differ in cost but it looks fishy to me. The ALGO clinics spent more on medications. That was offset by there being less spent on "nonphysician outpatient services" [?]. These comparisons don’t answer the questions raised by the suit obviously. Both TAU and ALGO used the same formulary. And both groups used the Atypicals heavily over the first generation drugs. But it does point out that the Schizophrenia arm of the Texas Medical Algorithm Project was much ado about nothing in terms of improving the lot of the patients.

I recall that clozapine made its début in the 1970s, thanks to Hanns Hippius in Munich. The problem with agranulocytosis prevented clozapine from entering general use, but it definitely gained a reputation as an effective drug for refractory patients with schizophrenia. By the 1980s, clozapine also was being talked up as a treatment for tardive dyskinesia (TD). That is one important reason why, when the rest of the ‘atypical’ antipsychotic drugs appeared in the 1990s, we all assumed, with little evidence, that they also would have a low propensity to cause TD. That assumption was a major force in the early popularity of the ‘atypicals’ and TMAP seems to have banked on it (excuse the pun).
Now we know in the clear light of today that the rate of TD with ‘atypicals’ is not very different from the rate with the early antipsychotic drugs. A current, prospective study that makes this point is: SW Woods et al. Journal of Clinical Psychiatry 2010;71:463-474. It took us 20 years to wake up to the reality of TD with the original antipsychotic drugs, so perhaps we improved slightly on that record with the ‘atypicals.’
My personal campaign has been against the casual use of ‘atypicals’ in primary care settings to treat so-called refractory depression, because patients with primary diagnoses of mood disorders seem to be even more susceptible than schizophrenic patients are to develop TD, while at he same time the relative benefit to the depressed patients is much less clear.
Just remember this little pearl I learned in pharmacy school:
all drugs follow the 4 P’s of pharmacological agenda, that being promise, panacea, placebo, and poison.
Placebo does not infer true comparison to a sugar pill, but, to the established predecessors to the new drug that all the pros/cons are known, and per the attitude of the new drug on the block, the predecessors are basically presented as placebos. So, comparing second generation antipsychotics to Haldol was basically the same premise.
As far as panacea, just look how every second generation antipsychotic has been marketed after getting approval for treating schizophrenia.. How naive I was to think that Astra Zeneca would be more responsible in marketing Seroquel.. In my opinion, they have put Lilly to shame in their zealous, arrogant marketing of Seroquel as the second coming of sliced bread!!!
It is fascinating to see all drugs follow this pattern. And they do. And anyone who argues otherwise has an agenda to either protect their product or their service.
It is nothing less than insincere and disingenuous to argue otherwise.
Hope this comment is of interest to the blog and thread.
Both points well made. The “4 P’s” phrase is new to me. Watch me steal that line down the road!
Do you know of any good attorneys for cases against Clozaril. My daughter has been taking it for several years and developed chronic heart and other illnesses.