 m]
m] In 1985, Jobson contacted a group of colleagues to share algorithms. The Delphi method – each participant’s treatment sequences would be submitted, then all shared among the group, minimizing the influence of “expert” opinions – was used. This “project” was well-received and informative. Eventually the group – which included faculty from Duke, Emory, Harvard, Stanford and Yale Universities; National Institutes of Mental Health and multiple international sites including the Universities of Vienna and Stellenbosh [South Africa] and from Sendai, Japan – addressed, one by one, the major Axis I psychiatric illnesses in this way.
In 1992, speaking with longtime friend and colleague Bill Potter, then head of extra-mutual research at NIMH, Jobson learned that there was virtually no interest in funding research about medication choice sequencing [algorithms]. Jobson explained that clinicians make those decisions daily and spoke about his informal algorithm project. They agreed that it would be worthwhile to have a national conference to create awareness of the need for psychopharmacology algorithms. So the project was formalized as the International Psychopharmacology Algorithm Project and the first educational conference was held in 1993 at the National Institutes of Mental Health.
The Texas Medication Algorithm Project: Development and Implementation of the Schizophrenia Algorithm
by John A. Chiles, Alexander L. Miller, M. Lynn Crismon, A. John Rush, Amy S. Krasnoff, and Steven S. Shon
Psychiatric Services 50:69-74, 1999.The Texas Medication Algorithm Project is a program designed to improve the quality of care of persons with serious mental disorders across sites in the Texas public mental health system and to create a uniform clinical environment from which cost estimates can be made. This paper describes the process of developing a pharmacological treatment algorithm for schizophrenia that addresses use of antipsychotics as well as other medications for side effects and co-existing symptoms. Input from clinicians, consultants, and consumers informed development of the algorithm, which was based on existing expert consensus guidelines. Information about the project can be found on the Internet at [LINK]. The authors present and describe the original and current versions of the algorithm, outline the feedback process by which it will be refined, and discuss how new medications will be incorporated as they enter the market.
Acknowledgments: This project is supported by grants from the Robert Wood Johnson Foundation, Meadows Foundation, Moody Foundation, Nannie Hogan Boyd Charitable Trust, Texas Department of Mental Health and Mental Retardation, Center for Mental Health Services, National Institute of Mental Health (grant MH-53799), Bristol-Myers Squibb Company, Eli Lilly and Company, Glaxo-Wellcome, Inc., Janssen Pharmaceutica, Novartis Pharmaceuticals Corporation, Pfizer, Inc., and Wyeth-Ayerst Laboratories, as well as Mental Health Connections, a partnership between the Dallas County Department of Mental Health and Mental Retardation and the department of psychiatry at the University of Texas Southwestern Medical School. Funding is also provided by the Texas State Legislature and the Dallas County Hospital District.
The expert consensus guideline series: treatment of schizophrenia
by Frances A, Docherty JP, and Kahn DA
Journal of Clinical Psychiatry 57(suppl 12B):1-58, 1996.
The expert consensus guideline series: treatment of schizophrenia 1999
by McEvoy JP, Scheifler PL, and Frances A
Journal of Clinical Psychiatry 60 (suppl 11)::3-80.1999.
Acknowledgements: This Guide was prepared by Peter J. Weiden, M.D., Patricia L. Scheifler, M.S.W., Joseph P. McEvoy, M.D., Allen Frances, M.D., and Ruth Ross, MA. M.A. The guide includes recommendations contained in the 1999 Expert Consensus Treatment Guidelines for Schizophrenia. The Editors gratefully acknowledge Laurie Flynn and the National Alliance for the Mentally Ill for their generous help and permission to adapt their written materials. Eli Lilly, Janssen Pharmaceutica, Novartis Pharmaceuticals, Ortho-McNeil Pharmaceutical, Pfizer Inc, and Zeneca Pharmaceuticals provided unrestricted educational grants in support of this project.
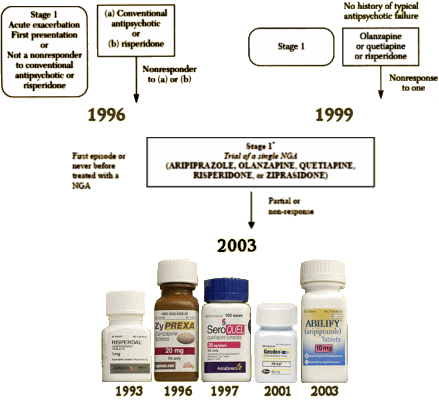
Algorithm development and implementation took place in the fall of 1996 and systematically involved input from groups of clinicians, consultants, and consumers. Early in this process, the participants agreed to five general principles governing the development of the schizophrenia algorithm.
•The goals of the algorithm are to improve quality of care in the Texas public mental health system, to create a uniform clinical environment from which cost estimates can be made, and to provide data to inform revisions in the algorithm.•The algorithm provides a sequential framework for clinical decision making. When possible, multiple options are available at a given stage so that the treatment plan can be tailored for optimal outcomes. Subsequent treatment is informed by the patient’s response. Critical decision points define movement between stages.•The DSM-IV diagnostic framework is necessary but not sufficient to establish the boundaries of the algorithm. Patients with chronic psychotic disorders for whom antipsychotic medications are the mainstay of treatment are the target population. Psychotic mood disorders are excluded, because they are addressed in the TMAP algorithm for major depressive disorder.•Use of the algorithm for a given patient is determined by the physician’s decision to change medication. A physician may initiate treatment for a patient at any stage of the algorithm.•Participating physicians are required to use the algorithm or state their reason in a progress note for not doing so. These reasons become part of the database for revisions to the algorithm.For several reasons, the initial algorithm for schizophrenia was limited to pharmacotherapy. First, the literature on medications provides significant evidence on which to base clinical consensus. Second, although medications constitute a small portion of the mental health budget, the increased costs of new medications have caused administrative scrutiny and, in many cases, have resulted in limitations on physicians’ prescribing choices. Third, appropriate pharmacotherapy may decrease utilization of crisis services and increase the demand for psychosocial rehabilitation programs. Data from implementation of the medication algorithm might inform the development of uniformly applied, consensus-driven, psychosocial rehabilitation programs.
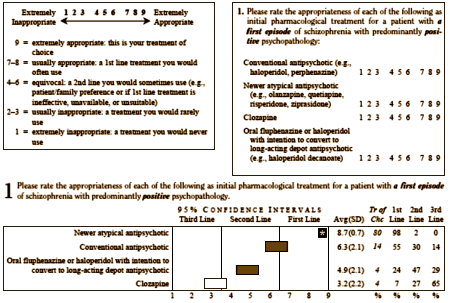
After the survey results were analyzed and ratings assigned, the next step was to turn these recommendations into user friendly guidelines. For example, the results of the question presented above are shown on p. 35 and are used in Guideline 1: Initial Treatment for an Acute Episode [p. 12]. Newer atypical antipsychotics appear as the treatment of choice for first episode schizophrenia with predominantly positive symptoms [treatments of choice are indicated by bold italics in the guidelines], while conventional antipsychotics are a high second line option.
What we didn’t know in 1999 [at least I didn’t know] was that every Physician listed in this post so far [except yours truly] was in some way compromised by having financial connections with one or usually more of the pharmaceutical companies that manufactured these drugs [including many of the 57 experts]; that every study used to get these drugs approved by the FDA had significant methodological inconsistencies; that there were a number of unreported studies that contradicted the good news about the Atypical Antipsychotics; that our literature was flooded with ghost-written articles full of manipulated scientific information; in short, that the great wave of evidence-based medicine in 1999 was an illusion, actively created by the pharmaceutical industry, with willing participation by the upper echelons of academic and organized psychiatry.
A pair of researchers from the University of North Carolina at Chapel Hill will investigate the effectiveness of a new class of antipsychotic drugs under one of the largest National Institutes of Health awards ever made.
The $42.1 million contract, spread over five years, is expected to be announced today by the National Institute of Mental Health, a unit of the NIH, the nation’s premiere medical-research agency. The contract is the largest ever awarded by the unit and ranks among the largest ever awarded by the entire NIH, according to university and agency officials.
The research, which will be conducted at 50 clinical sites around the country, is designed to test the effectiveness and safety of a relatively new class of antipsychotic drugs referred to as atypicals. The drugs are on the market – including Novartis AG’s Clozaril, Johnson & Johnson’s Risperdal, Eli Lilly & Co.’s Zyprexa and AstraZeneca PLC’s Seroquel – and have turned into major sellers.
link to 2010 revision version of harvard psychopharmacology algorithm
http://www.evidence2011.com/sites/default/files/posters/Osser_BipolarDepressionAlgorithm.pdf
“evidence-based medicine in 1999 was an illusion, actively created by the pharmaceutical industry” –that destroyed my family as we knew it.