A Double-blind, Randomized, Placebo-Controlled Trial of Fluoxetine in Children and Adolescents With Depression
by Graham J. Emslie, MD; A. John Rush, MD; Warren A. Weinberg, MD; Robert A. Kowatch, MD; Carroll W. Hughes, PhD; Tom Carmody, PhD; and Jeanne Rintelmann
Archives of General Psychiatry. 1997, 54:1031-1037.Abstract
Background: Depression is a major cause of morbidity and mortality in children and adolescents. To date, randomized, controlled, double-blind trials of antidepressants (largely tricyclic agents) have yet to reveal that any antidepressant is more effective than placebo. This article is of a randomized, double-blind, placebo controlled trial of fluoxetine in children and adolescents with depression.
Method: Ninety-six child and adolescent outpatients (aged 7-17 years) with nonpsychotic major depressive disorder were randomized (stratified for age and sex) to 20 mg of fluoxetine or placebo and seen weekly for 8 consecutive weeks. Randomization was preceded by 3 evaluation visits that included structured diagnostic interviews during 2 weeks, followed 1 week later by a 1-week, single-blind placebo run-in. Primary outcome measurements were the global improvement of the Clinical Global Impressions scale and the Children’s Depression Rating Scale – Revised, a measure of the severity depressive symptoms.
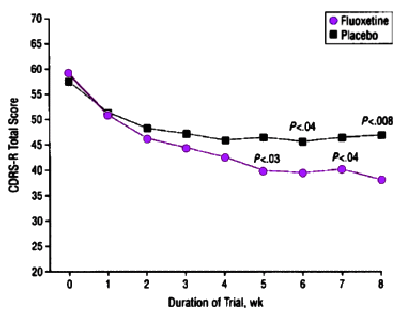
Results: Of the 96 patients, 48 were randomized to fluoxetine treatment and 48 to placebo. Using the intent to treat sample, 27 (56%) of those receiving fluoxetine and 16 (33%) receiving placebo were rated "much" or "very much" improved on the Clinical Global Impressions scale at study exit (chi 2=5.1, df=1, P=.02). Significant differences were also noted in weekly ratings of the Children’s Depression Rating Scale – Revised after 5 weeks of treatment (using last observation carried forward). Equivalent response rates were found for patients aged 12 years and younger (n=48) and those aged 13 years and older (n=48). However, complete symptom remission (Children’s Depression Rating Scale – Revised) occurred in only 31% of the fluoxetine-treated patients and 23% of the placebo patients.
Conclusion: Fluoxetine was superior to placebo in the acute phase treatment of major depressive disorder in child and adolescent outpatients with severe, persistent depression. Complete remission of symptoms was rare.
Efficacy of Paroxetine in the Treatment of Adolescent Major Depression: A Randomized, Controlled Trial
by MARTIN B. KELLER, M.D., NEAL D. RYAN, M.D., MICHAEL STROBER, PH.D., RACHEL G. KLEIN, PH.D., STAN P. KUTCHER, M.D., BORIS BIRMAHER, M.D., OWEN R. HAGINO, M.D., HAROLD KOPLEWICZ, M.D., GABRIELLE A. CARLSON, M.D., GREGORY N. CLARKE, PH.D., GRAHAM J. EMSLIE, M.D., DAVID FEINBERG, M.D., BARBARA GELLER, M.D., VIVEK KUSUMAKAR, M.D., GEORGE PAPATHEODOROU, M.D., WILLIAM H. SACK, M.D., MICHAEL SWEENEY, PH.D., KAREN DINEEN WAGNER, M.D., PH.D., ELIZABETH B. WELLER, M.D., NANCY C. WINTERS, M.D., ROSEMARY OAKES, M.S., AND JAMES P. MCCAFFERTY, B.S.
Journal of the American Academy of Child and Adolescent Psychiatry, 2001, 40(7):762–772.
AbstractObjective: To compare paroxetine with placebo and imipramine with placebo for the treatment of adolescent depression.Method: After a 7 to 14-day screening period, 275 adolescents with major depression began 8 weeks of double-blind paroxetine [20–40 mg], imipramine [gradual upward titration to 200–300 mg], or placebo. The two primary outcome measures were endpoint response [Hamilton Rating Scale for Depression [HAM-D] score <8 or >50% reduction in baseline HAM-D] and change from baseline HAM-D score. Other depression-related variables were [1] HAM-D depressed mood item; [2] depression item of the Schedule for Affective Disorders and Schizophrenia for Adolescents-Lifetime version [K-SADS-L]; [3] Clinical Global Impression [CGI] improvement scores of 1 or 2; [4] nine-item depression subscale of K-SADS-L; and [5] mean CGI improvement scores.Results: Paroxetine demonstrated significantly greater improvement compared with placebo in HAM-D total score <8, HAM-D depressed mood item, K-SADS-L depressed mood item, and CGI score of 1 or 2. The response to imipramine was not significantly different from placebo for any measure. Neither paroxetine nor imipramine differed significantly from placebo on parent- or self-rating measures. Withdrawal rates for adverse effects were 9.7% and 6.9% for paroxetine and placebo, respectively. Of 31.5% of subjects stopping imipramine therapy because of adverse effects, nearly one third did so because of adverse cardiovascular effects.Conclusions: Paroxetine is generally well tolerated and effective for major depression in adolescents.

Fluoxetine for Acute Treatment of Depression in Children and Adolescents: A Placebo-Controlled, Randomized Clinical Trial
by GRAHAM J. EMSLIE, M.D., JOHN H. HEILIGENSTEIN, M.D., KAREN DINEEN WAGNER, M.D., PH.D., SHARON L. HOOG, M.D., DANIEL E. ERNEST, B.A., EILEEN BROWN, PH.D., MARY NILSSON, M.S., AND JENNIE G. JACOBSON, PH.D.
Journal of the American Academy of Child and Adolescent Psychiatry, 2002, 41(10):1205–1215.
Abstract
Method: After a 3-week screening period, 122 children and 97 adolescents with MDD (DSM-IV) were randomly assigned to placebo or fluoxetine. After a 1-week placebo lead-in, fluoxetine-treated patients received fluoxetine 10 mg/day for 1 week, then fluoxetine 20 mg/day for 8 weeks.
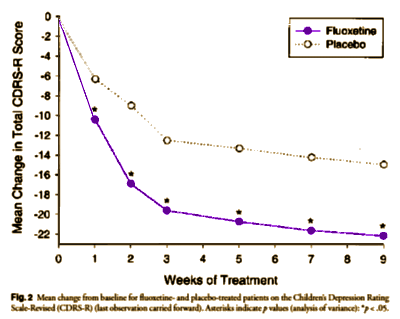
Results: Fluoxetine was associated with greater mean improvement in Children’s Depression Rating Scale-Revised (CDRS-R) score than placebo after 1 week (p < .05) and throughout the study period. Significantly more fluoxetine-treated patients (41%) met the prospectively defined criteria for remission than did placebo treated patients (20%) (p < .01). More fluoxetine- (65%) than placebo-treated (53%) patients met the prospectively defined response criterion of ≥30% decrease in CDRS-R score, but this difference was not significant (p = .093). Significantly more fluoxetine- than placebo-treated patients completed acute treatment (p = .001). There were no significant differences between treatment groups in discontinuations due to adverse events (p = .408).
Conclusion: Fluoxetine 20 mg daily appears to be well tolerated and effective for acute treatment of MDD in child and adolescent outpatients. Fluoxetine is the only antidepressant that has demonstrated efficacy in two placebo-controlled, randomized clinical trials of pediatric depression.
Efficacy of sertraline in the treatment of children and adolescents with major depressive disorder: two randomized controlled trials
by Wagner KD, Ambrosini P, Rynn M, Wohlberg C, Yang R, Greenbaum MS, Childress A, Donnelly C, Deas D; and the Sertraline Pediatric Depression Study Group
Journal of the American Medical Association. 2003, 290(8):1033-41.
AbstractCONTEXT: The efficacy, safety, and tolerability of selective serotonin reuptake inhibitors (SSRIs) in the treatment of adults with major depressive disorder (MDD) are well established. Comparatively few data are available on the effects of SSRIs in depressed children and adolescents.
OBJECTIVE: To evaluate the efficacy and safety of sertraline compared with placebo in treatment of pediatric patients with MDD.
DESIGN AND SETTING: Two multicenter randomized, double-blind, placebo-controlled trials were conducted at 53 hospital, general practice, and academic centers in the United States, India, Canada, Costa Rica, and Mexico between December 1999 and May 2001 and were pooled a priori.
PARTICIPANTS: Three hundred seventy-six children and adolescents aged 6 to 17 years with Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition-defined MDD of at least moderate severity.
INTERVENTION: Patients were randomly assigned to receive a flexible dosage (50-200 mg/d) of sertraline (n = 189) or matching placebo tablets (n = 187) for 10 weeks.
MAIN OUTCOME MEASURES: Change from baseline in the Children’s Depression Rating Scale-Revised (CDRS-R) Best Description of Child total score and reported adverse events.
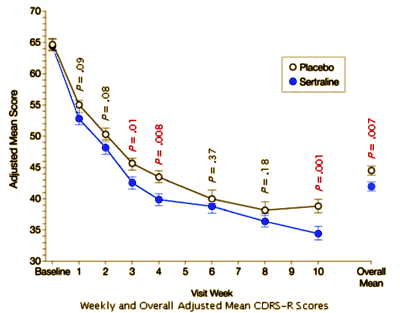
RESULTS: Sertraline-treated patients experienced statistically significantly greater improvement than placebo patients on the CDRS-R total score (mean change at week 10, -30.24 vs -25.83, respectively; P =.001; overall mean change, -22.84 vs -20.19, respectively; P =.007). Based on a 40% decrease in the adjusted CDRS-R total score at study end point, 69% of sertraline-treated patients compared with 59% of placebo patients were considered responders (P =.05). Sertraline treatment was generally well tolerated. Seventeen sertraline-treated patients (9%) and 5 placebo patients (3%) prematurely discontinued the study because of adverse events. Adverse events that occurred in at least 5% of sertraline-treated patients and with an incidence of at least twice that in placebo patients included diarrhea, vomiting, anorexia, and agitation.
CONCLUSION: The results of this pooled analysis demonstrate that sertraline is an effective and well-tolerated short-term treatment for children and adolescents with MDD.


My old professor would’ve gone on from there to locate any number of jury rigged features to make these trivial differences shrink even more – things like how drop-outs were handled [particularly the Zoloft study]. Actually, even with the full articles in hand, there are so many obfuscations and missing facts that it’s hard to know if there are any actual differences at all. Oh yeah, none of these articles are forthcoming about the suicidal ideation that emerged along the way.
-
Fluoxetine 20 mg daily appears to be well tolerated and effective for acute treatment of MDD in child and adolescent outpatients
-
Paroxetine is generally well tolerated and effective for major depression in adolescents
-
Fluoxetine was superior to placebo in the acute phase treatment of major depressive disorder in child and adolescent outpatients with severe, persistent depression
-
The results of this pooled analysis demonstrate that sertraline is an effective and well-tolerated short-term treatment for children and adolescents with MDD
So we have a quartet of trials, all shaky, all massaged, all financed by the pharmaceutical company that makes the drug, all written by people ‘on the payroll,’ at least one ghost-written, drugs that we know are ineffective for the advertised condition, all four claiming safety and efficacy. I’m sure my old professor wouldn’t believe what he was reading. What can we do about this state of affairs? The Internet is plenty big – lot’s of room. They could post their raw data so the rest of us could check it out. In fact, if someone has ‘good data,’ we would be glad to tell the world of its goodness.
Ah, but such beautiful lies! Look here, primary care doc… we know you don’t know nothing about depression,. so here’s the deal: it’s called fluoxetine and the dose is 20 mg and it’s once a day and there are no side effects and it works and so now you don’t need to feel inadequate in managing depression any more so you can go back to worrying about hypertension and asthma.
And to think, the mass drugging of children with SSRIs over the last decade, from infancy through adolescence, was in large part based on the trials listed above, rigged to make it look like the drugs worked. The only side effect ever mentioned is suicide but in reality who knows how much damage was done to the developing bodies of all these children?
Like you Dr Nardo, I don’t believe the vast majority of the kids that were drugged had any mental disorder to begin with. I also have a lot of experience working with children who had plenty of reasons to be unhappy and I never recommended that a single one to be put on drugs.
The Emslie trials had to resort to a placebo run-in to get any where NEAR the results required to stretch the numbers into the appearance of efficacy. In earlier trials, placebo out-performed the active drug. The Emslie trials started ALL participants on placebo and dropped the “early responders†after 2 weeks and THEN began the comparison trials. I understand that the placebo run-in became standard practice, but I cannot imagine why it is called objective science.
“The only side effect ever mentioned is suicide but in reality who knows how much damage was done to the developing bodies of all these children?
Like you Dr Nardo, I don’t believe the vast majority of the kids that were drugged had any mental disorder to begin with. I also have a lot of experience working with children who had plenty of reasons to be unhappy and I never recommended that a single one to be put on drugs.”
AMEN Evelyn! Everything you said rang true, as I was dosed with SSRIs starting at age 14 for what was, in hindsight, perfectly understandable situational anxiety over my dad’s cancer diagnosis. And for the first eight years or so, the meds did absolutely NOTHING. They didn’t help my anxiety and they didn’t give me side effects. But starting in the 8th year when my doctor added Effexor to my Lexapro (yeah, I know, TWO SSRIs! I didn’t know it at the time but I was lucky I didn’t get serotonin syndrome), I started developing disturbing new side effects, like lowered libido, reduced pleasure in orgasm, decreased genital sensitivity, decreased reaction to erotic visual stimuli (it’s a frightening, alien feeling to be on a college campus and see attractive girls and feel like I’m looking at a piece of cardboard), anhedonia, and a terrible — and bizarre — social anxiety that’s completely uncharacteristic of me.
I’m currently tapering off my Lexapro and will hopefully be off this time next year. It’s hard not to drown in uncertainty: will I be able to withdraw from SSRIs once and for all? What withdrawal symptoms may I have? How severe will they be? Will I regain my emotional response and my sexual functioning, or will I develop PSSD (Post-SSRI-Sexual Dysfunction), in which people still have sexual dysfunction even after they’re off their meds for YEARS? I don’t think I have to tell anyone it’s totally unacceptable psychiatry hasn’t even begun research to START to answer these questions that 1000s (perhaps millions worldwide) of us have when in this situation.
Oh, and I DID try to get off the SSRIs about ten years ago but couldn’t as my psychiatrist’s withdrawal protocol was much too fast and gave me terrible withdrawal symptoms (brain zaps, head pressure, etc) that left me bedridden. When I went back into his office screaming for an explanation, he just looked puzzled and came up with only one suggestion: go back on the medication. That was the first time in my life that I was truly scared and wondered, just like you, what damage would be done to my developing body/mind.
I must say that psychiatry has caused me to lose a lot of faith not just in medicine, but in this country. The sense of betrayal scalds deep. It’s a terrible feeling to have for someone in their 20s, but here I am. I constantly ask myself, “What’s the point of being a good person and trying to forge a career when criminal slobs like Biederman, Nemeroff, Trivedi, Schatzberg, et. all don’t follow the rules, make millions off their shoddy ‘research’ which causes suffering to many (often children), and then just get a slap on the wrist?” It’s just so demoralizing, but this is the trend in this country, and it’s not just relegated to psychiatry.
SG – Reading your post validated some of my worst fears about what might be happening to kids who were fed these drugs. I fully realize that there are millions more like you and I am so sorry that this was allowed to happen. Thank you for sharing your story.
Evelyn —
Thank you so much for your reply. All I can say is my generation of kids who “came of age” on psych meds (many of them unnecessarily) are some of the strongest people in this country today. It is utterly harrowing to come to terms with the facts that:
a) The psychiatric establishment doesn’t give a damn about you or your devastating side effects that you may have to live with for many years (or perhaps forever), and that you were just dollar signs for big pharma
b) Any research into just what causes terrible things like PSSD or prolonged anhedonia is most likely years (if not decades) away, and only IF the psychiatric and scientific research community FINALLY gets the message about the damage these meds do and how tremendously wrong they were in prescribing these meds so widely
c) I’m somehow going to have to come up with motivation and other “emotions” through sheer will-power in order to make a good impression with potential employers because, at least for right now, I have tremendous difficulty accessing these necessary emotions, which of course makes everything in my life 100x more difficult (in fact, I lost my first romantic relationship to my anhedonia and sexual dysfunction — can you imagine what it feels like for an otherwise healthy 22-year-old to lose their first relationship to drug-induced sexual and mental dysfunction?). And, of course, trying to forge a new career in the worst economy since the Depression doesn’t help.
I guess I’m just sick and tired of being strong all the time. And mad. Yeah, I get mad a lot too.
Allen,
That’s why these studies are a quartet. They are neither “objective” nor “science.”
Here are the background documents (all linked) how Prozac came to be approved for children in Europe – UK and Sweden was responsible:
http://jannel.se/prozac-children-partII.pdf
Thought it would be interesting to know that the Dutch Medicines Evaluation Board (Rapporteur) had the following to say far into the process:
“In the face of the limited efficacy results, safety concerns are all the more salient. Increased risk for suicide related behaviours emerged as the most concerning safety finding from the clinical trials. Other safety concerns include effects on growth and sexual maturation including effects on fertility, and effects on cognitive and emotional development.â€
It’s an exciting story, almost unbelievable.
As a historical note, the original tricyclic antidepressant drugs were tested for depression in children in the late 1970s and early 1980s. The lead investigator was Joaquim (Kim) Puig-Antich. The main drug tested was imipramine (Tofranil). The trials were run at Columbia and later at U. Pittsburgh. Kim was never able to demonstrate efficacy in depressed children, and he was not afraid to say so. If imipramine was not effective, then there is little reason to think that the SSRI class of drugs would be, either, because they are generally viewed as antidepressants lite.