As promised, the US Justice Department – along with the US Securities and Exchange Commission – is paying closer attention to interactions between the pharmaceutical industry and foreign governments. In recent months, at least five big drugmakers have received letters as the federal government seeks to uncover any violations of the Foreign Corrupt Practices Act, which forbids US companies from bribing foreign government officials…
Among the countries cited are Brazil, China, Germany, Greece, Italy, Poland, Russia and Saudi Arabia, according to Main Justice, which notes that the Arnold & Porter law firm recently issued a client advisory indicating the probe is also exploring whether drugmakers and clinical trial organizations pay off third-party investigators to finesse research data. Why? A recent report by the HHS Office of Inspector General noted that eight percent of marketing applications to the FDA in 2008 relied exclusively on foreign data, 80 percent of approved marketing applications for drugs and biologics relied on at least one foreign clinical study, and 78 percent of all human subjects were enrolled at foreign sites.
“The number of third party contracts with CROs, academic and health care facilities, and investigators provides a high-risk area for potential violations. The US government is particularly interested in any corrupt payments that may have wrongfully influenced the reliability or integrity of data emerging from any trial, and companies may find themselves facing critical legal issues if approval of products rested on the results of studies that DOJ deems corrupt,” Arnold & Porter attorneys wrote, adding the “DOJ has recently taken the public position that many investigators, foreign clinicians, and laboratory workers will be deemed ‘foreign officials’ for purposes of the FCPA”…
Looking over the ACRO [Association of Clinical Research Organizations] web-site, it seems to be standard industry-specific trade organization fare. Their central thrust is Globalization. In fact the policy page seems organized around the topic:
ACRO’s global advocacy program is focused around a set of core principles:
• Secure a consistent, stable regulatory environment to support the continued globalization of clinical research• Protect the safety of research participants globally• Expand participation in clinical trials by physician investigators and volunteers; special emphasis on minority and special populations• Ensure ease of access to data necessary to conduct research while considering patient privacy• Promote industry participation in comparative effectiveness research• Encourage health information technology policies that include consideration of medical research needs• Advance policies that encourage innovation in pharmaceutical, biologic and regenerative medicine development• Support a strong FDA with expanded resources in the areas of regulatory science and international operations• Support the EMA and European Commission in their efforts to advance GCP compliance and enhance international inspection regimes• Oppose conflict of interest policies that unduly restrict research and innovation• Further efforts to promote translational research through NIH and other government-funded bodiesIn fact, they have a White Paper on Globalization [downloadable if you give them a name and email address]:
Executive Summary:As clinical trials have become increasingly globalized over the past ten to fifteen years, the possibility of conducting studies that offer adequate subject protection and yield reliable results in emerging countries has understandably attracted considerable attention. In this analysis, we examine the facts regarding the current state of clinical research and the role that biopharmaceutical companies and their clinical research organization (CRO) partners play in ensuring that the dual goals of trial safety and quality are met. Although concerns have been raised about the globalization of biomedical research, the reality is that emerging countries play a vital role in the advancement of medical science. Clinical trials in these countries, particularly those with industry sponsorship, are conducted at the high standards necessary to obtain regulatory approval in major markets. In addition, the investments made by trial sponsors, which are frequently implemented by CROs, are a major contributor to improving the health systems and economies of the developing world…

from Deadly Medicine
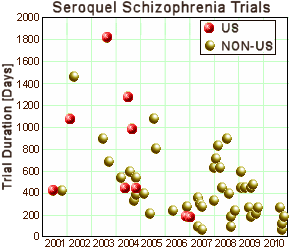
I put it together to show that sending trials overseas allowed them to shorten the time it took to complete a study, but it shows something else – that globalization is a reality. Given the inflated efficacy and questionable safety of Seroquel, it wouldn’t be hard to convince me that AstraZeneca paid for their questionable results.
The following headline may be of interest:
Gardasil: efficacité non démontrée en prévention du cancer du col de l’utérus, dit la revue Prescrire
And this :
Arrêt d’un essai clinique du Gardasil en Inde: 7 décès, 120 effets indésirables graves, conflits d’intérêts, désinformation…
Both are recent articles in Pharmacritique
Gardasil was introduced as a cure for cancer, and per my memory, immediately pushed for a mandate that included children not included in the original sample. Texas medical officials took the step of informing the legislature of their concerns, since this was going to be passed without medical input.
In my very first sales class I was taught that persistence was one of the keys to success. Pharma has learned this lesson well, and will use whatever means works, time and time again to market a drug, worthwhile or not.
I view psychiatry as the test bed for pharma’s goal of taking over all of medicine to promote its products and standards. As a small part of medicine it becomes the perfect test subject. First we control the testing. Then we move on to a political imitative to promote our product to the public, pushing politicians to cover the cost and we support the whole process with bought and paid for KOL’s.
The recent revelations regarding the prescribing of antidepressants without fitting the medical criteria is an example of this marketing strategy at work. Like the headlines above, pharma will control the flow of information to its advantage. We have yet to see this information in an English format.
Lack of effectiveness, death, injury, conflicts of interest and disinformation are not topics we will see regarding a drug in the US, until the legal cases are filed.
Steve Lucas
The corruption is almost mind numbing to those that follow this endless wave of permeating corporate crime.
Steve that was an interesting article, even if my french linguistic understanding is limited to counting up to 10 and oui….