Professor McGorry defends early intervention
Australian Broadcasting Corporation
Reporter: Tony Jones
August 18, 2011
TONY JONES: One of the harshest of those critics is an American psychiatrist, Professor Alan Frances. He calls Australia’s early intervention programs the "… largest and most reckless public health experiment ever attempted." I mean, it’s a pretty extravagant criticism. How do you respond to that?
PATRICK MCGORRY: Well, it’s quite bizarre. Dr Frances has obviously no knowledge of the Australian health care system nor of the processes that these reform proposals have had to go through to get to this stage. I mean, it’s been – we’ve debated these issues in the journals, in the health reform commissions, we’ve even debated them on the beaches, I would say. I mean, it’s been a massive effort to actually get it through all these lenses.
So I think what we’re seeing now is some disaffected critics who, as David Crosby said, are not happy with the result. They’ve had their chance to put the case; the case hasn’t got up from their point of view. I’ve been a researcher in this area for 20 years, I’m a scientist; this is not advocacy, this is not faith, this is absolutely hard evidence. These programs improve outcomes and they reduce costs. So an early psychosis program will end up costing per patient per annum about a third of what the standard adult mental health care costs. So actually these reforms release money for other areas of psychiatry, so it’s very self-defeating for people to criticise their implementation.
TONY JONES: I was trying to figure out why an American would be amongst your strongest critics, and it seems that Professor Frances and others are quite worried that what you’re doing here in Australia will entrench a newly-described state of mental illness known as "psychosis risk syndrome". Is what you’re doing here really that revolutionary that it could affect the way psychiatry is practiced all around the world?
PATRICK MCGORRY: Well, my colleague Alison Yung and I in the early days of our early psychosis work in the ’90s, patients were presenting to the first episode psychosis programs with lot of distress, a lot of functional impairment, but with warning signs of psychosis. So, these patients were in need of some kind of assistance. So what we set about doing was first of all following up these patients in a supportive way and we learned that they had a very high risk of transitional to psychosis, something like 30 to 40 per cent within 12 months, which is very, very high, about 400 times higher than the general population.
And so obviously there was a need to try to reduce that risk, so a number of research studies, which I think Jon Jureidini referred to, were conducted both here and overseas. There are about six randomised control trials, if he wants to talk evidence, showing that a range of treatments will reduce that risk to about 10 per cent. Now, the sort of things that work in reducing risk are cognitive behaviour therapy, a psychological treatment, omega three fatty acids, a fish oil, both of which studies we have done, and low-dose anti-psychotic medication. Now that’s the controversial bit.
But what the studies actually show is there is no need to use anti-psychotic medications as first line in these patients. So the fears that Dr Francis is fanning in this country are actually the converse of what the reality actually shows from the research. So, I think we’re on very firm ground knowing how to help these young people and they certainly need help. And they shouldn’t be turned away, as Dr Jureidini implied. They should be helped and they can be helped within this new blend of Headspace and EPPIC models which will be rolled out hopefully to most of Australia over the next few years…
Randomized controlled trial of interventions designed to reduce the risk of progression to first-episode psychosis in a clinical sample with subthreshold symptoms.
by McGorry PD, Yung AR, Phillips LJ, Yuen HP, Francey S, Cosgrave EM, Germano D, Bravin J, McDonald T, Blair A, Adlard S, and Jackson H.
Arch Gen Psychiatry. 2002 59(10):921-8.
BACKGROUND: Most disability produced by psychotic illnesses, especially schizophrenia, develops during the prepsychotic period, creating a case for intervention during this period. However, only recently has it been possible to engage people in treatment during this phase.
METHODS: A randomized controlled trial compared 2 interventions in 59 patients at incipient risk of progression to first-episode psychosis. We termed this group ultra-high risk to emphasize the enhanced risk vs conventional genetic high-risk studies. Needs-based intervention was compared with specific preventive intervention comprising low-dose risperidone therapy (mean dosage, 1.3 mg/d) and cognitive behavior therapy. Treatment was provided for 6 months, after which all patients were offered ongoing needs-based intervention. Assessments were performed at baseline, 6 months, and 12 months.
RESULTS: By the end of treatment, 10 of 28 people who received needs-based intervention progressed to first-episode psychosis vs 3 of 31 from the specific preventive intervention group (P=.03). After 6-month follow-up, another 3 people in the specific preventive intervention group became psychotic, and with intention-to-treat analysis, the difference was no longer significant (P=.24). However, for risperidone therapy-adherent patients in the specific preventive intervention group, protection against progression extended for 6 months after cessation of risperidone use.
CONCLUSIONS: More specific pharmacotherapy and psychotherapy reduces the risk of early transition to psychosis in young people at ultra-high risk, although their relative contributions could not be determined. This represents at least delay in onset (prevalence reduction), and possibly some reduction in incidence.
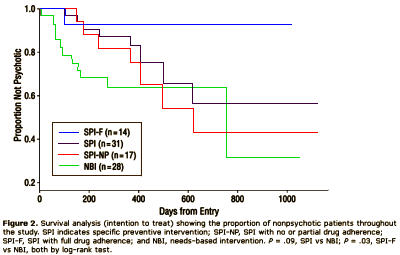
In this first study, financed in part by Janssen-Cilag Pharmaceuticals [a subdivision of Johnson & Johnson], they had 59 subjects identified as qualifying for their "ultra high risk" group. There were three study groups. The first group was fofflowed with "Needs-Based Intervention" [NBI] which was traditional supportive psychotherapy and case management. The second group was treated with "Specific Prevention Intervention" [SBI] which combined the standard therapy [NBI] plus a particular vesion of Cognitive Behavior Threapy [CBT] developed for these "pre-psychotic" patients plus Risperidal 1.0 – 2.0 mg/day. In these two groups, SPI was continued for six months, then both groups were followed with NBI only for another six months.
| GROUP | N | PSYCHOTIC | |
| 6 months [%] | 1 year [%] | ||
|
|
|||
| NBI | 28 | 10 [36%] | 10 [36%] |
| SPI | 31 | 3 [10%] treatment |
6 [19%] |
|
|
|||
| p=0.03 | p=0.24 | ||
So the significance disappeared at 1 year. When they looked at the SPI Group, all of the late converters were in a group with poor medication compliance [SPI-NP, none or partial] so this is the graph they published:
There were several other confounding variables as well. I refer you to their full text on-line if you’re interested.
Randomized controlled trial of interventions for young people at ultra-high risk of psychosis: study design and baseline characteristics. .
by Phillips LJ, Nelson B, Yuen HP, Francey SM, Simmons M, Stanford C, Ross M, Kelly D, Baker K, Conus P, Amminger P, Trumpler F, Yun Y, Lim M, McNab C, Yung AR, McGorry PD.
Aust N Z J Psychiatry. 2009 43(9):818-29.
OBJECTIVE: Intervention during the pre-psychotic period of illness holds the potential of delaying or even preventing the onset of a full-threshold disorder, or at least of reducing the impact of such a disorder if it does develop. The first step in realizing this aim was achieved more than 10 years ago with the development and validation of criteria for the identification of young people at ultra-high risk (UHR) of psychosis. Results of three clinical trials have been published that provide mixed support for the effectiveness of psychological and pharmacological interventions in preventing the onset of psychotic disorder.METHOD:The present paper describes a fourth study that has now been undertaken in which young people who met UHR criteria were randomized to one of three treatment groups: cognitive therapy plus risperidone (CogTher + Risp: n = 43); cognitive therapy plus placebo (CogTher + Placebo: n = 44); and supportive counselling + placebo (Supp + Placebo; n = 28). A fourth group of young people who did not agree to randomization were also followed up (monitoring: n = 78). Baseline characteristics of participants are provided.RESULTS AND CONCLUSION: The present study improves on the previous studies because treatment was provided for 12 months and the independent contributions of psychological and pharmacological treatments in preventing transition to psychosis in the UHR cohort and on levels of psychopathology and functioning can be directly compared. Issues associated with recruitment and randomization are discussed.
Randomized controlled trial of interventions for young people at ultra high risk for psychosis: 6-month analysis.
by Yung AR, Phillips LJ, Nelson B, Francey SM, PanYuen H, Simmons MB, Ross ML, Kelly D, Baker K, Amminger GP, Berger G, Thompson AD, Thampi A, and McGorry PD.
Journal of Clinical Psychiatry. 2011 72(4):430-40.
OBJECTIVE: Cognitive therapy and/or low-dose antipsychotic administered during the prodromal phase of schizophrenia may prevent or delay the onset of full-blown illness. However, it is unclear which of these treatments are most effective, how long treatment should be given, and whether effects will be sustained over a prolonged period.METHOD: In order to examine these issues, we conducted a randomized controlled trial of cognitive therapy + risperidone; cognitive therapy + placebo; and supportive therapy + placebo in young people at ultra high risk for developing a psychotic disorder (that is, putatively prodromal). The main outcome was transition to psychotic disorder, with level of symptoms and functioning the secondary outcomes. This article reports the interim 6-month follow-up results. The study was conducted from August 2000 to May 2007.RESULTS: Of a possible 464 eligible ultra high risk individuals, 115 were recruited to the randomized controlled trial (cognitive therapy + risperidone, n = 43; cognitive therapy + placebo, n = 44; and supportive therapy + placebo, n = 28). An additional 78 individuals agreed to follow-up assessments but not to randomization ("monitoring group," n = 78). At 6 months, 8 of the 115 participants (7.0%) and 4 of the monitoring group (5.1%) had developed psychotic disorder. There were no significant differences between the 3 randomized groups (log rank test, P = .92) or between all 4 groups (log rank test, P = .93). There was also no difference between the 4 groups in secondary measures, with all groups showing a reduction in symptoms and increased functioning.CONCLUSIONS: Rates of transition to psychosis were lower than expected, particularly in the control supportive therapy + placebo group. This may have accounted for the negative finding, as the sample was therefore underpowered to find any difference between groups. Alternatively, it may be that all treatments were equally effective or equally ineffective at 6 months.
The first article was a "prequel" [published two years after the completion of the study] explaining the design – obviously tailored to definitively answer questions about the earlier 2002 study. They had three treatment groups: Cognitive Therapy + Risperdal [43], Cognitive Therapy + Placebo [44], and Supportive Therapy + Placebo [28]. They also had a "monitoring" group with no intervention [78].
a randomized, placebo-controlled trial.
by Amminger GP, Schäfer MR, Papageorgiou K, Klier CM, Cotton SM, Harrigan SM, Mackinnon A, McGorry PD, and Berger GE.
Archives of General Psychiatry. 2010 67(2):146-54.[full text on-line]
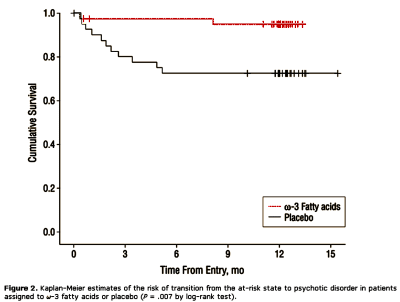
CONTEXT: The use of antipsychotic medication for the prevention of psychotic disorders is controversial. Long-chain omega-3 (omega-3) polyunsaturated fatty acids (PUFAs) may be beneficial in a range of psychiatric conditions, including schizophrenia. Given that omega-3 PUFAs are generally beneficial to health and without clinically relevant adverse effects, their preventive use in psychosis merits investigation.OBJECTIVE: To determine whether omega-3 PUFAs reduce the rate of progression to first-episode psychotic disorder in adolescents and young adults aged 13 to 25 years with subthreshold psychosis.DESIGN: Randomized, double-blind, placebo-controlled trial conducted between 2004 and 2007.SETTING: Psychosis detection unit of a large public hospital in Vienna, Austria.PARTICIPANTS: Eighty-one individuals at ultra-high risk of psychotic disorder.
INTERVENTIONS: A 12-week intervention period of 1.2-g/d omega-3 PUFA or placebo was followed by a 40-week monitoring period; the total study period was 12 months.MAIN OUTCOME MEASURES: The primary outcome measure was transition to psychotic disorder. Secondary outcomes included symptomatic and functional changes. The ratio of omega-6 to omega-3 fatty acids in erythrocytes was used to index pretreatment vs posttreatment fatty acid composition.RESULTS: Seventy-six of 81 participants (93.8%) completed the intervention. By study’s end (12 months), 2 of 41 individuals (4.9%) in the omega-3 group and 11 of 40 (27.5%) in the placebo group had transitioned to psychotic disorder (P = .007). The difference between the groups in the cumulative risk of progression to full-threshold psychosis was 22.6% (95% confidence interval, 4.8-40.4). omega-3 Polyunsaturated fatty acids also significantly reduced positive symptoms (P = .01), negative symptoms (P = .02), and general symptoms (P = .01) and improved functioning (P = .002) compared with placebo. The incidence of adverse effects did not differ between the treatment groups.CONCLUSIONS: Long-chain omega-3 PUFAs reduce the risk of progression to psychotic disorder and may offer a safe and efficacious strategy for indicated prevention in young people with subthreshold psychotic states. Trial Registration clinicaltrials.gov Identifier: NCT00396643.
Sorry, the comment form is closed at this time.