As long as we’re on the subject of psychiatrists working behind the scenes, I’d like to return to the topic of Dr. Patrick McGorry who is in the center of the push for early intervention in an "ultra high risk" of adolescents and young adults who go on to develop Schizophrenia at a greater rate than others [the magnitude of that rate varies from study to study and from investigator to investigator]. For review, the controvery centers around:
-
elevating this "ultra high risk" group to a "disorder" in the DSM-5, vigorously opposed by Dr. Allen Frances and others
-
using Antipsychotics to treat a group for a disease that only a minority will ever develop
-
A massive expenditure for an early intervention program without solid proof of its effectiveness
The mental health policy [3] of Australia is not my business. I’m not in love with the DSM-IIIs or DSM-IVs, so I doubt I’ll be enamored with the DSM-5 no matter what they do, but adding this category sure seems way premature to me [2]. I personally think giving Atypical Antipsychotics to a group who might develop Schizophrenia borders on malpractice – first because it is exposing mostly non-psychotic people to unnecessary danger and second because there’s no real proof that early intervention with medication will even help the people long term that do become psychotic [1]. It’s "an experimental hypothesis" at best, not "a program" or even "a treatment." But that said, I think the idea of early, intensive intervention in Schizophrenia is a great concept to be worked towards.
Since I’ve already said these things repeatedly, why am I back on the topic? First, someone sent me
an editorial written by Dr. McGorry himself almost a decade ago that says some of what I just said in a softer way, concluding:
Deinstitutionalization was a wonderful reform idea which in many places resulted in perverse outcomes because it was not properly evaluated or resourced. Early intervention should not make the same mistake.
It’s a fine editorial, worthy of your full reading [as a matter of fact, McGorry might reread it himself].
But the second thing is something that came my way that is in need a bit of an introduction. Looking at Dr. McGorry’s group’s work on early intervention, there were four studies of note [
1. when n=a few…]:
-
-
-
-
That last study [4] gave this rationale for the treatment:
Based on findings of reduced long-chain  -3 and
-3 and  -6 polyunsaturated fatty acids [PUFAs] in individuals with schizophrenia, it has been argued that dysfunctional fatty acid metabolism could be involved in the etiology of the disorder. Four controlled trials in people with schizophrenia have found beneficial effects of
-6 polyunsaturated fatty acids [PUFAs] in individuals with schizophrenia, it has been argued that dysfunctional fatty acid metabolism could be involved in the etiology of the disorder. Four controlled trials in people with schizophrenia have found beneficial effects of  -3 supplementation, while 2 other studies reported negative findings, and 2 recent meta-analyses reported that results remain inconclusive. The therapeutic effects of
-3 supplementation, while 2 other studies reported negative findings, and 2 recent meta-analyses reported that results remain inconclusive. The therapeutic effects of  -3 PUFAs may result from altered membrane fluidity and receptor responses following their incorporation into cell membranes.
-3 PUFAs may result from altered membrane fluidity and receptor responses following their incorporation into cell membranes.  -3 Polyunsaturated fatty acids may also interact with the dopaminergic and serotonergic systems, which both have been associated with the pathophysiology of schizophrenia through modulation of receptor-coupled arachidonic acid release. Furthermore, eicosapentaenoic acid, an
-3 Polyunsaturated fatty acids may also interact with the dopaminergic and serotonergic systems, which both have been associated with the pathophysiology of schizophrenia through modulation of receptor-coupled arachidonic acid release. Furthermore, eicosapentaenoic acid, an  -3 PUFA, may increase glutathione in the temporal lobes of first-episode psychosis patients. There are data to suggest that glutathione may be low in schizophrenia and protects neurons from excitotoxicity and oxidative stress, which is documented in schizophrenia. The evidence that
-3 PUFA, may increase glutathione in the temporal lobes of first-episode psychosis patients. There are data to suggest that glutathione may be low in schizophrenia and protects neurons from excitotoxicity and oxidative stress, which is documented in schizophrenia. The evidence that  -3 PUFAs can reduce symptoms in schizophrenia, may have neuroprotective properties, and do not have clinically relevant adverse effects make them an ideal candidate for indicated prevention in young people at risk of psychosis, in whom the use of antipsychotic medication is controversial. Thus, we sought to determine whether
-3 PUFAs can reduce symptoms in schizophrenia, may have neuroprotective properties, and do not have clinically relevant adverse effects make them an ideal candidate for indicated prevention in young people at risk of psychosis, in whom the use of antipsychotic medication is controversial. Thus, we sought to determine whether  -3 PUFA can [1] prevent a first episode of psychotic disorder and [2] reduce psychiatric symptoms and improve functioning in individuals with subthreshold manifestations of psychosis.
-3 PUFA can [1] prevent a first episode of psychotic disorder and [2] reduce psychiatric symptoms and improve functioning in individuals with subthreshold manifestations of psychosis.
I have no knowledge base to understand this rationale, much less critique it. I reproduce it here for the learned, but mainly to show that they had a rationale. My read is that they’re saying that PUFAs are benign and that there’s some reason to think they might work, so why not give it a shot? The results looked pretty good:
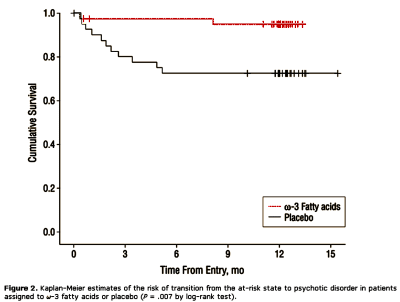
[recolored for clarity]
The next part of the story is that people [including me] were up in arms because Dr. McGorry had registered a Clinical Trial of the Atypical Antipsychotic Seroquel in his high risk group. The trial was cancelled recently, but McGorry claimed it wasn’t because of the uproar, it was because he’d decided in favor of another trial of the PUFAs instead of Seroquel [the internal critic…].
Which brings us to this patent application:
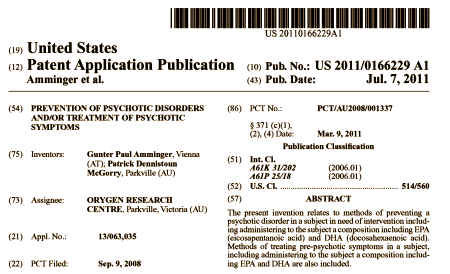
I’m not much up on the way patent applications work, but as best I can tell, this patent was filed on September 9, 2008:
Inventors: Amminger, Gunter Paul (Vienna, AT) and Mcgorry, Patrick Dennistoun (Parkville, AU)
Application Number: 13/063035
Publication Date: 07/07/2011
Filing Date: 09/09/2008
Assignee: ORYGEN RESEARCH CENTRE (Parkville, Victoria, AU)
View Patent Images: Download PDF 20110166229
That was some three months before the
article above [4] was submitted for publication for the first time [
December 18, 2008]. The
pdf in the patent application has the graphs from the publication [to be]:
AUTHOR INFORMATION
Correspondence: G. Paul Amminger, MD…
Submitted for Publication: December 18, 2008; final revision received May 26, 2009; accepted May 27, 2009.
Financial Disclosure: None reported.
Funding/Support: This study was supported by grant 03T-315 from the Stanley Medical Research Institute.
Author Contributions: Dr Amminger had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis; all authors have seen and approved the final version.
Role of the Sponsors: The sponsor of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report.
Additional Contributions: Dr Sarah E. Hetrick and Professors Michael Berk and Anthony F. Jorm provided helpful comments on this manuscript; Dr Sherilyn Goldstone edited the final manuscript; Magdalena Holub, MSc, Ingrid Holzer, MSc, Margit Kornsteiner, PhD, and Jessica Slavik, MSc, assisted with the erythrocyte fatty acid analysis. We thank all of the participants and their families.
Author Affiliations: Department of Child and Adolescent Psychiatry, Medical University of Vienna, Vienna, Austria (Drs Amminger, Schäfer, Papageorgiou, and Klier); Orygen Research Centre, Centre for Youth Mental Health, The University of Melbourne, Melbourne, Australia (Drs Amminger, Cotton, Mackinnon, and McGorry and Ms Harrigan); and Department of Research and Education, The Schlössli Clinic, Oetwil am See, Switzerland (Dr Berger).
I believe this
Clinical Trial registration is the one for this study, but when I look at the dates the trial was registered, the submission and publication dates for the article, and the patent application dates – the timelines just don’t work out. I hope someone can get this muddle untangled. I had to put it aside for a clearer moment.
This whole thing doesn’t look very good to me. It sure looks like a conflict of interest that should’ve been declared – reporting on a study where you’ve applied for a patent on the drug being studied. It doesn’t look right to me to be holding the reins on a massive governmental public health initiative [EPPIC] in which you mention a treatment with a drug you’re trying to patent yourself without making the patent application public. It has the feel of the same kind of sheenanigans that we’ve endured here in the US for the last decade with psychopharmacology – stealthy entrepreneurialism.
I don’t know the original source for either the editorial or the patent information. They were passed on to me by friends because I’ve been writing about McGorry, but they’re both readily available in the public domain. The editorial is clarifying and from McGorry in a wiser moment. The patent application is something McGorry should’ve been telling us about all along. I tried to see if a patent had been applied for in other countries [ie Australia], but that’s outside my Internet expertise. Someone else with the know-how should take a look. This is the kind of thing that has given the specialty of psychiatry a really bad name, and it needs to stop. The medical principle is that anything that gives the appearance of a conflict of interest should be declared. Beyond that, it undermines the lofty and crusading rhetoric that accompanies Dr. McGorry’s appeals…
 -3 and
-3 and  -6 polyunsaturated fatty acids [PUFAs] in individuals with schizophrenia, it has been argued that dysfunctional fatty acid metabolism could be involved in the etiology of the disorder. Four controlled trials in people with schizophrenia have found beneficial effects of
-6 polyunsaturated fatty acids [PUFAs] in individuals with schizophrenia, it has been argued that dysfunctional fatty acid metabolism could be involved in the etiology of the disorder. Four controlled trials in people with schizophrenia have found beneficial effects of  -3 supplementation, while 2 other studies reported negative findings, and 2 recent meta-analyses reported that results remain inconclusive. The therapeutic effects of
-3 supplementation, while 2 other studies reported negative findings, and 2 recent meta-analyses reported that results remain inconclusive. The therapeutic effects of  -3 PUFAs may result from altered membrane fluidity and receptor responses following their incorporation into cell membranes.
-3 PUFAs may result from altered membrane fluidity and receptor responses following their incorporation into cell membranes.  -3 Polyunsaturated fatty acids may also interact with the dopaminergic and serotonergic systems, which both have been associated with the pathophysiology of schizophrenia through modulation of receptor-coupled arachidonic acid release. Furthermore, eicosapentaenoic acid, an
-3 Polyunsaturated fatty acids may also interact with the dopaminergic and serotonergic systems, which both have been associated with the pathophysiology of schizophrenia through modulation of receptor-coupled arachidonic acid release. Furthermore, eicosapentaenoic acid, an  -3 PUFA, may increase glutathione in the temporal lobes of first-episode psychosis patients. There are data to suggest that glutathione may be low in schizophrenia and protects neurons from excitotoxicity and oxidative stress, which is documented in schizophrenia. The evidence that
-3 PUFA, may increase glutathione in the temporal lobes of first-episode psychosis patients. There are data to suggest that glutathione may be low in schizophrenia and protects neurons from excitotoxicity and oxidative stress, which is documented in schizophrenia. The evidence that  -3 PUFAs can reduce symptoms in schizophrenia, may have neuroprotective properties, and do not have clinically relevant adverse effects make them an ideal candidate for indicated prevention in young people at risk of psychosis, in whom the use of antipsychotic medication is controversial. Thus, we sought to determine whether
-3 PUFAs can reduce symptoms in schizophrenia, may have neuroprotective properties, and do not have clinically relevant adverse effects make them an ideal candidate for indicated prevention in young people at risk of psychosis, in whom the use of antipsychotic medication is controversial. Thus, we sought to determine whether  -3 PUFA can [1] prevent a first episode of psychotic disorder and [2] reduce psychiatric symptoms and improve functioning in individuals with subthreshold manifestations of psychosis.
-3 PUFA can [1] prevent a first episode of psychotic disorder and [2] reduce psychiatric symptoms and improve functioning in individuals with subthreshold manifestations of psychosis.

Sorry, the comment form is closed at this time.