Having looked at the Risperdal Augmentation trial [Effects of risperidone augmentation in patients with treatment-resistant depression:…] with an eye to its various permutations as it was published and republished, then looked at its shrinking conclusions[racketeer influenced and corrupt organizations…], I got kind of curious about the study itself. What had they set out to prove in the first place [hypothesis]? Why were the conclusions so garbled? plastic?
They took only antidepressant treatment failures into the study and treated them [again?] with an SSRI [Citalopram] for a month to six weeks in an open-label, non-placebo controlled trial. A few responded [12%]. Then they treated the non-responders with the SSRI [Citalopram] and added Risperdal for another month to six weeks. Of this group, 70% responded. All of this was the prequel to the study itself – impossible to evaluate because it was open-label with no placebo control.
The actual study itself was a double-blind trial in which the SSRI non-responders who had responded when Risperdal was added were either continued on Risperdal or not with their SSRI for six months. Pretty confusing? This table says it again [the study is in blue]:
|
Subjects were in- or outpatients, aged 18–85 years, DSM-IV diagnostic criteria for major depressive disorder, single or recurrent episode, with or without psychotic features, and with a HAM-D-17 >20. Subjects were required to have a history of resistance to standard antidepressant therapy, defined as failure to respond to at least one but not more than three adequate antidepressant trials during the current episode.
|
|
Open-label citalopram monotherapy: … Patients who were nonresponders (<50% reduction in HAM-D-17) at the end of citalopram monotherapy were eligible to enter the risperidone augmentation phase of the study.
|
|
Open-label risperidone augmentation: … Patients who achieved symptom resolution, defined as a HAM-D-17 score >7 or CGI-S < 2, with risperidone augmentation were eligible to enter the double-blind continuation phase.
|
|
Double-blind continuation phase: In the 24-week double-blind phase, patients were randomized to continue on risperidone plus citalopram or to receive placebo plus citalopram. The primary outcome was time to relapse, defined as any one or more of these four criteria:
[1] CGI-Change (CGI-C) > 6 [2] HAM-D-17 >16 [3] discontinuation owing to lack of therapeutic effect [4] deliberate self-injury or suicidal intent |
What were they looking to find? If the responding patients who were continued on Risperdal did better that the ones who weren’t, they could’ve recommended that continuing Risperdal for six months would be a good idea. Had both groups done equally well, they could’ve said, you don’t have to keep giving Risperdal as a chronic treatment. It got them over the hump!
Here are the results as best as I can extract them from the information given:
| PHASE | START | WITHDREW | RESPOND | NON-RESPOND |
|
| I [open] | citalopram | 489 | 44 | 55 | 434 |
| II [open] | risperidone citalopram |
386 | 38 | 243 | 105 |
| NON-RELAPSE | RELAPSE | ||||
| III [blind] | placebo citalopram |
119 | 11 | 43 | 65 |
| III [blind] | risperidone citalopram |
122 | 14 | 43 | 65 |
In the actual study, they got nothing – neither a difference nor both groups doing well, ergo a failed study. Actually, the long term results showed that the two groups did equally badly, each with a 60% relapse rate. I don’t think that was in the plan.
And so they went back and looked at the replapse rates during the continuation period and found that the group who had been the least responsive during Phase I [Citalopram] and who had responded in Phase II [Risperdal Augmentation] had a slowed time to relapse in Phase III in the Placebo Group as opposed to the Risperdal Group [p < 0.05] though the difference in relapse rates was insignificant [p = 0.40] [after the Corrigendum].
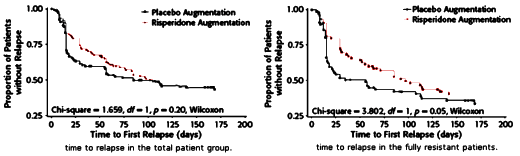
In Dr. Carroll’s letter, he questions even this difference, but just by looking at the graphs, there’s not much there to write home about no matter what the statistics show. Frankly, I’m not completely sure what they wanted to show in this study, unless they thought it might show that you needed to take Risperdal chronically to prevent relapses. They certainly didn’t show that. The most they could legitimately say was something like, "In chronically depressed patients unresponsive to SSRIs, Risperdal Augmentation had a short-term effect [in an open-label, uncontrolled study], but those gains weren’t very sustainable, whether you continued Risperdal or not." But even that would’ve been a reach since there was no group with which to compare their results [as placebo treated patients traditionally do remarkably well in clinical trials]. The study was challenged from day one by a poor design out of the gate [little wonder that they published it a journal edited by one of the authors].
LOS ANGELES [August 4, 2006] – In the first large-scale study of its kind, researchers at Cedars-Sinai found that people suffering from resistant major depressive disorder who don’t respond to standard antidepressants can benefit when the drug therapy is augmented by a broad spectrum psychotropic agent, even when treated for a brief period of time. The study led by Mark HymanRapaport, M.D., chair of the department of psychiatry at Cedars-Sinai, was recently published in Neuropsychopharmacology AOP…
The study, which tracked a group of depressed patients who showed resistance to the standard SSRI antidepressant therapy found that those characterized as less severely ill only needed to stay on the broad spectrum psychotropic agent Risperidone for a brief period of time to experience lasting benefits; making their antidepressant medication work effectively. Those depressed patients who were characterized as more severely ill, however, needed to continue Risperidone on an ongoing basis to experience the same level of effectiveness…
I started this post because I couldn’t understand why this study was done in the first place. The design is just too odd. The part that nagged at me was Phase II. Why would they have done that part without a placebo or Citalopram + placebo control group? The place of Risperidal augmentation of antidepressants in treatment resistant depression was not already established. Why make the actual study continuance? Then I had an aha! moment and developed an immediate conspiracy theory [which I actually think might be the best explanation for why they conducted this study]. Had they just done an open-label, non-placebo-controlled short-term study, nobody would have even read it, if they could even get it published. By sticking the double blind continuance piece on the end, they could report a strong response to Risperidal augmentation in Phase II without having to deal with that pesky placebo comparison business – in fact, the placebo effect is on your side. The Phase III patients then become "relapsers" rather than a "non-responders." The trivial difference between the very sick and the sick then becomes something to spin [as above].
Risperidone for treatment-refractory major depressive disorder: a randomized trial
by Mahmoud RA, Pandina GJ, Turkoz I, Kosik-Gonzalez C, Canuso CM, Kujawa MJ, and Gharabawi-Garibaldi GM.
Annals of Internal Medicine 2007 147(9):593-602.
BACKGROUND: Major depressive disorder has high prevalence, morbidity, and mortality. Inadequate results with antidepressants have prompts addition of a nonstandard treatment (augmentation therapy).
OBJECTIVE: To assess whether augmentation therapy with risperidone reduces symptoms and increases response to antidepressant therapy and remission of depression in adults.
DESIGN: Multicenter, double-blind, placebo-controlled, randomized trial conducted from 19 October 2004 to 17 November 2005.
SETTING: 75 primary care and psychiatric centers.
PATIENTS: 274 outpatient adults with major depressive disorder that was suboptimally responsive to antidepressant therapy.
INTERVENTION: After a 4-week run-in period to ensure insufficient response to standard antidepressants, patients were randomly assigned to receive risperidone, 1 mg/d, or placebo for 6 weeks. After 4 weeks, the dosage of risperidone was increased to 2 mg/d in some cases.
MEASUREMENTS: Symptoms were measured by using the 17-item Hamilton Rating Scale for Depression (HRSD-17). Other outcomes were response to therapy, remission of depression, and various clinician- and patient-rated assessments.
RESULTS: Of the intention-to-treat population (268 patients), 81% (111 of 137) who received risperidone and 87.8% (115 of 131) who received placebo completed 6 weeks of double-blind treatment. Mean (+/-SE) HRSD-17 scores improved more in the risperidone augmentation group than in the placebo group (13.4 +/- 0.54 vs. 16.2 +/- 0.53; difference, -2.8 +/- 0.72 [95% CI, -4.2 to -1.4]; P <0.001). More risperidone recipients than placebo recipients experienced remission of depression (24.5% [26 of 106] vs. 10.7% [12 of 112]; P = 0.004) and had a response (46.2% [49 of 106] vs. 29.5% [33 of 112]; P = 0.004). Headache (8.8% of risperidone recipients vs. 14.5% of placebo recipients), somnolence (5.1% vs. 1.5%), and dry mouth (5.1% vs. 0.8%) were the most frequently reported adverse events.
LIMITATIONS: Patients were receiving many different antidepressants, and the duration of augmentation therapy was limited.
CONCLUSION: Risperidone augmentation produced a statistically significant mean reduction in depression symptoms, substantially increased remission and response, and improved other patient- and clinician-rated measures.
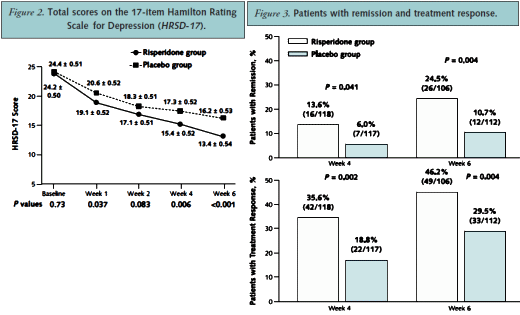
You saw the sleight of hand here very clearly, Mickey.
We all have to remember that corrupt organizations like Janssen-Rapaport-Nemeroff-Keller have great resources at their disposal to create these misleading infomercials. I would go so far as to ask whether the one Nemeroff published in the journal he edited, Neuropsychopharmacology, with Rapaport as lead author, didn’t actually cross the line into fraud.
Whatever; it requires something like perfect pitch to detect the deceits, and you have shown that you possess that skill.