Some echos just keep on reverberating, maybe until the end of days. I reckon the Atypicals are iterating towards becoming the Typicals, at least from the perspective of the F.D.A. [so much for there being a rule about there being a need before a new drug is introduced to the market]. But in psychopharmacology, making it onto the shelves is just the first step. After that, there’s a secondary pipeline – the indication march…
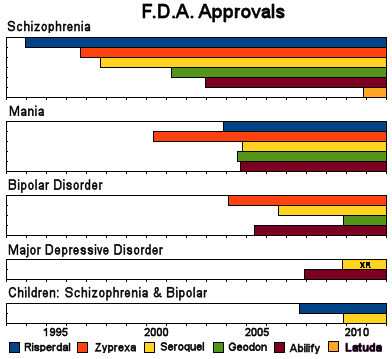
Latuda® has a rich tradition of Clinical Trials and marketing techniques to lead them on their journey. The first part was the hardest – getting on the board. That’s behind them now:
| Sunovion® Latuda Schizophrenia Clinical Trials | |||
| NCT ID | Recruit | Phase | StartDate |
|
|
|||
| NCT01074073 | Done | I | 08/2008 |
| NCT01082250 | Done | I | 07/2008 |
| NCT01074632 | Done | I | 05/2009 |
| NCT01082263 | Done | I | 10/2008 |
| NCT01082289 | Done | I | 09/2008 |
| NCT00044005 | Done | II | 09/2002 |
| NCT00088621 | Done | II | 07/2004 |
| NCT00044044 | Done | II | 07/2002 |
| NCT00088634 | Done | II | 05/2004 |
| NCT01435928 | Not Yet | III | 09/2011 |
| NCT00790192 | Done | III | 10/2008 |
| NCT00549718 | Done | III | 10/2007 |
| NCT00641745 | Done | III | 03/2008 |
| NCT00615433 | Done | III | 01/2008 |
| NCT01143090 | Active | III | 08/2010 |
| NCT01143077 | Done | III | 06/2010 |
| NCT00711269 | YES | III | 07/2008 |
Now comes the indication march proper [already in place well before the initial F.D.A. Approval]:
| Sunovion® Latuda Phase III Clinical Trials | |||
| NCT ID | Title | Recruit | StartDate |
|
|
|||
| NCT00868699 | 6-week Study, Bipolar I Depression (Monotherapy) | YES | 04/2009 |
| NCT00868959 | 24-week Extension Study, Bipolar I Depression | YES | 04/2009 |
| NCT00868452 | 6-week Study, Bipolar I Depression (Add-on) | YES | 04/2009 |
| NCT01284517 | 6-week Study, Bipolar I Depression | YES | 11/2010 |
| NCT01358357 | Bipolar Maintenance Adjunctive to Lithium or Divalproex | YES | 06/2011 |
| NCT01421134 | MDD With Mixed Features – Flexible Dose | YES | 09/2011 |
| NCT01423253 | MDD With Mixed Features – Extension | Not Yet | 09/2011 |
| NCT01423240 | MDD With Mixed Features | Not Yet | 10/2011 |
sharing information related to recurring topics of the boring old man
a medscape post on 9/28/11 that paints a detailed view of technology based diagnosis in psychiatry
Psychiatric Diagnosis in the Lab: How Far Off Are We?
Jeffrey A. Lieberman, MD
http://www.medscape.com/viewarticle/750288
Thanks for this link, jamzo. I sent a letter to Medscape about Dr. Lieberman’s program.
Regarding Dr. Jeffrey Lieberman’s program that aired 28 September 2011,”Psychiatric Diagnosis in the Lab: How Far Off Are We?”: This is the worst Medscape presentation I have ever seen. Dr. Lieberman reminds me of Rick Perry at the last Republican candidates’ debate – ill informed, fumbling, innumerate, with poverty of content of speech, lacking in specific information, and clueless to instruct on the process of biomarker development. Surely Medscape can do better! Please ensure that Dr. Lieberman receives this feedback.
It really was pretty bad, wasn’t it [Psychiatric Diagnosis in the Lab: How Far Off Are We?]? Of course, his venture in Tarrytown, NY [PsychoGenics Inc] may have something to do with his enthusiasm for laboratories to rise to the task at hand.
And don’t miss the Scientific Advisors. Eric Nestler from Mount Sinai is there [formerly the Chairman from the Texas Department that brought us STAR*D and TMAP]. Maurice Fava from Harvard [and STAR*D] is on board along with Nobel Laureate, Paul Greengard. And Columbia’s walk-about Chairman, Jeffrey Lieberman, is right there.
The trinity of evidence-based medicine, translational medicine, and personalized medicine are coming together just in time to meet the challenges of the patent expirations over the next few years. With an empty pharmaceutical pipeline, and the age of SSRIs and Atypical Antipsychotics coming to a close, a generation of neurobiologists is aiming to consummate the marriage of academic psychiatry to the pharmaceutical industry, and continue their lucrative march into a brave new world.
“The trinity of evidence-based medicine, translational medicine, and personalized medicine are coming together just in time to meet the challenges of the patent expirations over the next few years. With an empty pharmaceutical pipeline, and the age of SSRIs and Atypical Antipsychotics coming to a close, a generation of neurobiologists is aiming to consummate the marriage of academic psychiatry to the pharmaceutical industry, and continue their lucrative march into a brave new world.”
MIckey — I’m reminded, of all things, of the Solyndra debacle whenever you speak of psychiatric “science” that isn’t ready for prime time (personalized medicine, biomarkers, translational science, etc). Solyndra proved once and for all that you can’t just build a facility and wait for the Brinks trucks to pull up. The technology (here, solar power) has to be tested in the market first and be proven as financially viable/profitable before the government should lend one dime to it (if any money. I honestly don’t think the govt should subsidize any business costs).
But of course the White House didn’t do any of that and Solyndra went belly up.
Such is the case with Insel’s grand plan for what psychiatry will look like. It’s so obvious what psychiatry is doing: it’s scrambling for the next big thing to fill the vacuum formed from pharmaceutical companies’ growing absence. Are these “big things” thoroughly tested with real science and research? Of course not, as you have amply proved. But just like with Solyndra, appearance is reality, and as long as psychiatry appears that it’s moving forward, that’s all that matters (just as Obama made the appearance he was investing in the economy and creating jobs). And I’ll bet money that patients invested in the future of psychiatry will suffer more than layoffs like those in Solyndra for the folly.
Detestable.
Looks like Seroquel XR is going generic in 2016…