In nosing around about Paxil Withdrawal, I looked in PubMed for Author [Nemeroff + Schatzberg] – just wondering when they got to be such pals, and I ran across this article [full text on-line].
This article was accepted for publication in May 2006. At the time, Dr. Nemeroff was Chairman of Psychiatry at Emory and Editor of Neuropsychopharmacology, Dr. Keller was Chairman of Psychiatry at Brown, Dr. Charney was Dean for Academic and Scientific Affairs for Mount Sinai, and Dr. Schatzberg was Chairman of Psychiatry at Stanford. Dr. Kalali was VP for Medical and Scientific Services at Quintiles Transnational, and the remaining authors were his associates there.
Impact of Publicity Concerning Pediatric Suicidality Data on Physician Practice Patterns in the United States
by Charles B. Nemeroff, Amir Kalali, Martin B. Keller, Dennis S. Charney, Susan E. Lenderts, Elisa F. Cascade, Hugo Stephenson, and Alan F. Schatzberg
Archives of General Psychiatry. 2007 64(4):466-472.
Context: IMS Health Inc data presented by the Food and Drug Administration [FDA] on September 13 and 14, 2004, at a joint meeting of the Center for Drug Evaluation and Research’s Psychopharmacologic Drugs Advisory Committee and the FDA’s Pediatric Advisory Committee suggested that the number of children and teenagers who were prescribed antidepressants continued to increase in 2004, despite widespread publicity surrounding 2 FDA advisories regarding the potential for pediatric suicidality with selective serotonin reuptake inhibitor use. These results are contradictory to findings from the Medco Health Solutions, Inc, March 2004 analysis of pharmacy benefit claims and a separate subsequent analysis conducted by NDC Health using dispensing data from March 31, 2004, through June 30, 2005.
Objectives: To investigate the contradictory findings and provide additional analyses on the prescribing trends of antidepressants across age groups and physician specialties in the United States.
Design: Retail pharmacy prescription data and physician audit data were obtained from Verispan, a joint venture between Quintiles Transnational and McKesson. In addition to examining prescribing trends, a joinpoint regression analysis was conducted to identify the timing for significant changes in prescription use.
Results: The analyses suggest that the number of children and teenagers who were prescribed antidepressants has decreased significantly [P = .02] in the wake of widespread publicity surrounding the FDA public health advisories. Another impact of the advisories seems to be a shift in care from "generalists" to psychiatric specialists when it comes to prescribing antidepressants to patients younger than 18 years. Finally, the analyses highlight a slight shift in prescribing toward the non–selective serotonin reuptake inhibitor bupropion hydrochloride, even though it carries the same FDA "black box" warning as the selective serotonin reuptake inhibitors.
Conclusions: The effect on antidepressant prescribing volume observed in our analysis of the Verispan data parallels earlier findings reported by Medco Health Solutions, Inc, and NDC Health that the FDA actions have had a significant effect on the prescribing of antidepressants to children and adolescents. Together, these findings underline the importance of presenting a fair balance within the media due to the significant reach of this channel among prescribing physicians.
I usually leave off the Author’s Financial Disclosures, but in this case, they seemed pertinent:
Dr Nemeroff has received grants from or performed research for the American Foundation for Suicide Prevention, AstraZeneca, Bristol-Myers Squibb, Forest Laboratories, Inc, Janssen Pharmaceutica, NARSAD: The Mental Health Research Association, the National Institute of Mental Health, Pfizer Pharmaceuticals, and Wyeth-Ayerst Laboratories; has been a consultant to Abbott Laboratories, Acadia Pharmaceuticals, Bristol-Myers Squibb, Corcept Therapeutics, Cypress Bioscience, Cyberonics, Eli Lilly and Co, Entrepreneur’s Fund, Forest Laboratories, Inc, GlaxoSmithKline, i3 DLN, Janssen Pharmaceutica, Lundbeck, Otsuka America Pharmaceutical, Inc, Pfizer Pharmaceuticals, Quintiles Transnational, UCB Pharma, and Wyeth-Ayerst Laboratories; has been on the speakers bureau for Abbott Laboratories, GlaxoSmithKline, Janssen Pharmaceutica, and Pfizer Pharmaceuticals; is a stockholder in Acadia Pharmaceuticals, Corcept Therapeutics, Cypress Bioscience, and NovaDel Pharma Inc; is on the board of directors of the American Foundation for Suicide Prevention, the American Psychiatric Institute for Research and Education, the George West Mental Health Foundation, NovaDel Pharma Inc, and the National Foundation for Mental Health; holds patents on a method and devices for transdermal delivery of lithium [US 6,375,990 B1] and on a method to estimate serotonin and norepinephrine transporter occupancy after drug treatment using patient or animal serum [provisional filing April 2001]; and holds equity in Reevax, BMC-JR LLC, and CeNeRx.
Dr Kalali is on the advisory board or speakers bureau of AstraZeneca Pharmaceuticals LP, Bristol-Myers Squibb, GlaxoSmithKline, Janssen Pharmaceutica, Pfizer Inc, and Shire.
Dr Keller has been a consultant to or has received honoraria from Collegium, Cypress Bioscience, Cyberonics, Eli Lilly and Co, Forest Laboratories, Inc, Janssen Pharmaceutica, Organon, Otsuka America Pharmaceutical, Inc, Pfizer Inc, Pharmastar, Sepracor, Vela Pharmaceuticals Inc, and Wyeth Pharmaceuticals; has received grants from or performed research for Eli Lilly and Co, Forest Laboratories, Inc, Pfizer Inc, and Wyeth Pharmaceuticals; and has been on the advisory board of Abbott Laboratories, Bristol-Myers Squibb, Cyberonics, Cypress Bioscience, Eli Lilly and Co, Forest Laboratories, Inc, GlaxoSmithKline, Janssen Pharmaceutica, Novartis, Organon, Pfizer Inc, Sepracor, and Wyeth Pharmaceuticals.
Dr Charney has consulting agreements with Abbott Laboratories, AstraZeneca, Bristol-Myers Squibb, Cyberonics, Gene Logic Inc, the Institute of Medicine, Neurogen Corp, the Neuroscience Education Institute, Novartis Pharmaceuticals Corp, OREXIGEN Therapeutics, Inc, Organon International, Otsuka America Pharmaceutical, Inc, Quintiles Transnational, and Sepracore Inc; and has a confidentiality agreement with Forest Laboratories, Inc, and Novartis Pharmaceuticals Corp.
Dr Schatzberg is a consultant to Eli Lilly and Co, Wyeth Pharmaceuticals, Corcept Therapeutics, Bristol-Myers Squibb, Novartis, Abbott Laboratories, Forest Laboratories Inc, Quintiles Transnational, and Lundbeck; is a cofounder of Corcept Therapeutics and has equity in Forest Laboratories, Pfizer Inc, and Merck and Co; and has received research funding from GlaxoSmithKline and Wyeth Pharmaceuticals.
I suppose a good rule of thumb to take from this article is that if the Authors’ Financial Disclosures are longer than the Abstract itself, it means a change is coming in the authors’ careers. By August 2006, Dr. Nemeroff had "stepped down" as Editor of Neuropsychopharmacology in the wake of a scandal. Then came the 2008 probe of financial Conflicts of Interest and undeclared pharmaceutical income by Senator Grassley and investigator Paul Thacker. The list of offenders was long: Dr. Charles Nemeroff, Dr. Joseph Biederman, Dr. Melissa DelBello, Dr. Timothy Wilens, Dr. Thomas Spencer, Dr. Alan Schatzberg, Dr. Martin Keller, Dr. A. John Rush, Dr. Karen Wagner, Dr. Jeffrey Bostic, and Dr. Frederick Goodwin. Three of the above article’s authors were on that list and all are now no longer Chairmen of the same University [as all know, Dr. Nemeroff was hired at Miami after he ‘stepped down’ at Emory]. All four academic authors of the above article were included in the POGO expose` of ghost-writing. Dr. Dennis Charney, however, didn’t lose his position. In fact, he was promoted to Dean at Mount Sinai Medical School in 2007.
The context of this now five year old article is not completely clear as some of the prequel information isn’t available, but the gist of things is to look at impact of the 2004 addition of Black Box Warnings to antidepressants of suicidality in adolescents on these drugs. They reported on prescribing patterns for antidepressants in a large cohort:
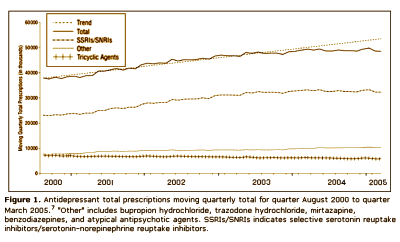
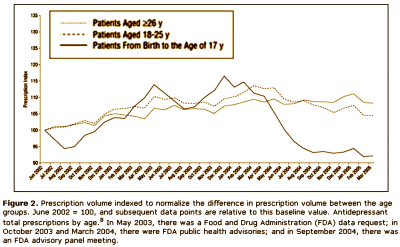
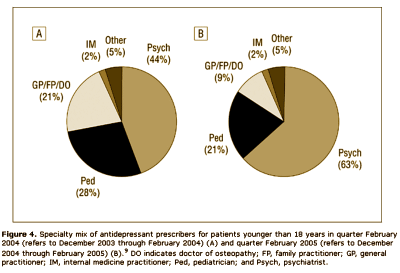
Looks good to me. Prescriptions down for adolescents and kids. Psychiatrists doing more of the prescribing. What’s not to like? I suppose I thought that they were doing this to prevent even more drastic measures, but when I read what they said, it seemed that they weren’t as pleased with things as I was:
US psychiatrists, as represented by the American Psychiatric Association and the American Academy of Child and Adolescent Psychiatry, have expressed concern that "the FDA action may limit access to necessary, appropriate, and effective treatment for children and adolescents with depression, anxiety, and other psychiatric disorders." This is especially interesting given that a previous preliminary study by the American College of Neuropsychopharmacology Task Force on SSRIs and Suicidal Behavior in Youth found no increase in suicidality among young patients taking SSRIs and other effective new-generation antidepressants, and this has been confirmed and extended in their final report…
In the current media environment in which safety concerns may be intensified because of several recent product recalls…, physician organizations (eg, the American Medical Association, the American Psychiatric Association, and the American Academy of Child and Adolescent Psychiatry) are concerned that the proved benefits of SSRI antidepressants may be underemphasized in discussions of potential risks and, as a result, there will be a decrease in access to appropriate treatment for children and adolescents. The FDA recently released results from an analysis that evaluated adult suicide and ideation data. The findings were mostly positive, and suggested that antidepressant drugs do not exacerbate suicidal thoughts in patients 30 years and older, but that the suicide thoughts/ideation seen in the pediatric data extends in young adults up to age 25 years. To date, these data results (both positive and negative) have received considerably less media attention in comparison with the release of the pediatric suicidality data. Recognizing that the results of the adult analysis were only public as of December 13, 2006, it remains to be seen if and how these findings will impact prescribing in both the 18 to 25 years population and the 26 years and older population. It is evident, however, that there is need for additional exploration into the relationship between FDA action, media reaction, and physician behavior change to ensure that dissemination of drug safety information does not interfere with appropriate access to care.
Well, I’d say that 2006 might be the apogee of corrupt psychiatry and this article marks the top of the curve. Three Department Chairmen and a Dean, none of whom are Child Psychiatrists, joining up with a Clinical Research Organization [Quintiles] to complain that the FDA warning of suicidality on antidepressants in children and adolescents [a group that doesn’t actually respond to these drugs in a meaningful way] has impacted drug sales. Talk about an abuse of power? This takes the cake. The most remarkable thing to me is that anyone would think that this particular group of authors would be believed as ombudsmen for the unmedicated children out of compassion for their suffering. They were the drug industry’s main go to guys. Even if you didn’t know what was coming as revelations about their sheenanigans, this would’ve been laughable at the time. Knowing what we know now, it’s ludicrous. Vagus Nerve Zappers? Corlux steroid blockers? Study 329? Complaining that kids aren’t getting enough drugs? Just too much. Where are the grown-ups?…
Just a quick note of appreciation for your blog which is never boring.
Where were the grown-ups? They were not in the editorial office or on the Editorial Advisory Board of Archives of General Psychiatry, that’s for sure.