Those of you who have been at this a while must giggle at people like me who are new to the scene as we discover things that you’ve known for a long time. I’ve been at this for a while now. I know that because I start looking something up, and find a link to something I personally already wrote, but hadn’t connected my new line of investigation. That happened yesterday. I was looking at the things Martin Keller signed onto after Study 329 [which was brought into question as soon as it was published in 2001] and I ran into the article below [again]. Here’s how I reproduced the abstract back in October [the apogee…]:
I usually leave off the Author’s Financial Disclosures, but in this case, they seemed pertinent:
|
At the time, I was focused on the fact that this article was accepted in May 2006, just a few months before Drs. Carrol and Rubin challenged Dr. Nemeroff’s article in the journal he edited [Neuropsychopharmacology] and Dr. Nemeroff ended up stepping down as editor because of undeclared conflicts of interest. Last month, I was thinking about Nemeroff’s hubris in publishing such a PHARMA-friendly article and the irony that all the authors in bold were later shown to have used ghost-writer Sally Laden and STI to write articles [paid by GSK][roaches…]. Yesterday [a walk on the wrong side side of the street?…], I was looking at the same article focusing on Martin Keller continuing to be a PHARMA sign-on lackey even after his poor show with Study 329. Now, I’m looking at the same article from yet another angle.
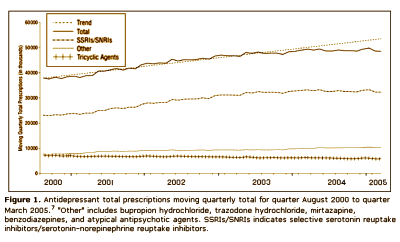
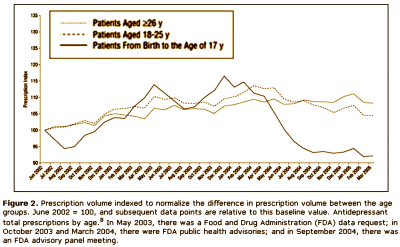
This article iteslf is actually a complaint against the FDA and the media. After years of haggling, the FDA finally had a hearing about the complaint that Paxil and the other SSRI’s could cause suicidality in some adolescents, and subsequently required warnings to be appended to these drugs for children and adolescents. Nemeroff et al are implying that the suicidality thing might have been overdone, and as a result, prescription rates had fallen dramatically in the younger age group. Here’s the kicker – their argument was that kids are being deprived of effective treatment because of the FDA and media. Unbelievable! But you already knew that.
So why are Dr. Nemeroff, Dr. Charney, Dr. Keller, and Dr. Schatzberg – all Department Chairmen of prestigious Departments of Psychiatry – writing this article? None of them are even Child and Adolescent Psychiatrists! I expect we would all suspect that they were PHARMA prostitutes, and that was why they were writing [or signing on to] this article. But who was getting paying them to do it? That lead me to get curious about those people in the author list I’d never heard of a month ago, but I was still too naive to look them up [so I put them in a background color] – Amir Kalali MD, … Susan E. Lenderts, Elisa F. Cascade, Hugo Stephenson MD.
The Journal Psychiatry is now called Innovations in Clinical Neuroscience. It’s a monthly on-line peer reviewed Journal published by Quintiles for psychiatrists. All of the "academic" authors above are on the Editorial Board.
1. MediGuard Safety Checks: Screening for drug-drug and drug-disease interactions.
2. Sending you email alerts and updates as important safety information arises for your medications.
3. Sharing feedback from other members on side effects and other important information.
4. Providing you with a printable list of your medications.
While MediGuard is an Internet "service" with 2.5 million subscribers. It is used by Quinitiles to track medication use for their marketing research – crossreferenced from patient records. Listen to the audio presentation by clicking on Elisa Cascade’s link.
One might say the apogee for the compromised KOLs was the nadir for the rest of us. Have we escaped the gravitational pull of Pharma and Pharma’s money? Not really. The journals that were corrupted by your cited examples have never publicly dissociated themselves from these compromised publications. The professional societies that installed Nemeroff, Charney, and Schatzberg as presidents have never disowned them, much less expelled them. The drug companies that enjoy corporate member status in these professional societies continue to be tolerated, despite the findings that many are guilty of felony criminal conduct, and most are guilty of plea bargained misdemeanor conduct for which billions of dollars in fines have been levied.
We still have a long way to go to put 2006 behind us.
This stuff goes back decades. Anyone who “told” what was going on was black balled.
I agree with Dr. Carroll; another aspect of this is how difficult it is going to be to change the mindset of the public wrt the medications. I think most of “the public” still believes psychotropic drugs are universally helpful, the normal course of treatment, have minimal side effects, aren’t addictive, and have proven efficacy. It is maddening.
I find it very interesting that Quintiles was behind the 2007 rant about the FDA’s black box warnings about antidepressants, since it is also the CRO that is putting together the deal that will allow drug companies to mine the medical records databases of 13 New York hospitals to recruit patients for their clinical trials. As I blogged about recently at http://alison-bass.com/blog, this partnership raises troubling privacy and ethical concerns.
Oops…forgot to mention, Mickey, we don’t giggle at you! We’re just grateful you’re here now to dig WAY below the surface!
Some professional societies suffer schisms when members decide not to renew memberships in protest. Why isn’t this happening to the American Psychiatric Association?
And how did Alan Schatzberg get elected president there anyway? What is the election process?