 After the post about Quintiles [an invisible empire II…], I recalled Amir Kalali’s name from somewhere else – the Latuda [Lurasidone] Trial I was writing about a few months back [ought to know by now…]. Over the Thanksgiving holiday while off visiting, I read it again for signs of Quintiles’ presence. Not much shows in the article itself. But this morning, I left Raleigh for the eight hour trek back to Georgia, and there it was! a big building in the Research Triangle Park sitting by the Interstate that said Quintiles on top. It was easier to find Quintiles on I-40 without even trying than it was to find them in the article. So I thought I’d look more closely:
After the post about Quintiles [an invisible empire II…], I recalled Amir Kalali’s name from somewhere else – the Latuda [Lurasidone] Trial I was writing about a few months back [ought to know by now…]. Over the Thanksgiving holiday while off visiting, I read it again for signs of Quintiles’ presence. Not much shows in the article itself. But this morning, I left Raleigh for the eight hour trek back to Georgia, and there it was! a big building in the Research Triangle Park sitting by the Interstate that said Quintiles on top. It was easier to find Quintiles on I-40 without even trying than it was to find them in the article. So I thought I’d look more closely:Lurasidone in the treatment of schizophrenia: a randomized, double-blind, placebo- and olanzapine-controlled study.
by Meltzer HY, Cucchiaro J, Silva R, Ogasa M, Phillips D, Xu J, Kalali AH, Schweizer E, Pikalov A, and Loebel A.
American Journal of Psychiatry. 2011 168[9]:957-67.
Objective: The study was designed to evaluate the short-term efficacy and safety of lurasidone in the treatment of acute schizophrenia.
Method: Participants, who were recently admitted inpatients with schizophrenia with an acute exacerbation of psychotic symptoms, were randomly assigned to 6 weeks of double-blind treatment with 40 mg of lurasidone, 120 mg of lurasidone, 15 mg of olanzapine (included to test for assay sensitivity), or placebo, dosed once daily. Efficacy was evaluated using a mixed-model repeated-measures analysis of the change from baseline to week 6 in Positive and Negative Syndrome Scale (PANSS) total score (as the primary efficacy measure) and Clinical Global Impressions severity (CGI-S) score (as the key secondary efficacy measure).
Results: Treatment with both doses of lurasidone or with olanzapine was associated with significantly greater improvement at week 6 on PANSS total score, PANSS positive and negative subscale scores, and CGI-S score compared with placebo. There was no statistically significant difference in mean PANSS total or CGI-S change scores for the lurasidone groups compared with the olanzapine group. With responders defined as those with an improvement of at least 20% on the PANSS, endpoint responder rates were significant compared with placebo for olanzapine only. The incidence of akathisia was higher with 120 mg of lurasidone (22.9%) than with 40 mg of lurasidone (11.8%), olanzapine (7.4%), or placebo (0.9%). The proportion of patients experiencing ≥7% weight gain was 5.9% for the lurasidone groups combined, 34.4% for the olanzapine group, and 7.0% for the placebo group.
Conclusions: Lurasidone was an effective treatment for patients with acute schizophrenia. Safety assessments indicated a higher frequency of adverse events associated with 120 mg/day of lurasidone compared with 40 mg/day.
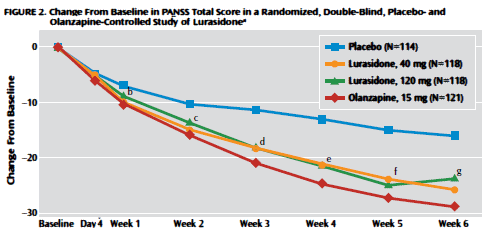
So first, about the multinational scope of the trial. This from clinicaltrials.gov, FDA documents, and the full text of the article:
| Country | Subjects | LUR40mg | LUR120mg | ZYP15mg |
|
|
||||
| US | 284 |
p=0.046 |
p=0.183 | p<0.001 |
| Lithuania | 29 | p=0.906 | p=0.188 | p=0.034 |
| Columbia | 48 | p=0.005 | p=0.017 | p=0.017 |
| Philippines | 26 | p=0.246 | p=0.911 | p=0.061 |
| India | 88 | |||
|
|
||||
| MMRM Analysis |
475 | p<0.001 |
p=0.011 | p<0.001 |
My reading of this data is that Lurasidone barely made significance in the US, and without the stellar results in Columbia, it would’ve been a wash overall. Multinational Clinical Trials are a hallmark of the Clinical Research Organizations [CROs]. For one thing, they’re faster [and, I suspect, cheaper]. You can read their position on globalization at the Association of Clinical Research Organizations [ACRO] web site. Also notice the unexpected level of significance in the MMRM Analysis [correction method for drop-outs].
Next, I wanted to see how close I could get to the raw PANSS totalscores. In the Online Data Supplement, there was a table of uncorrected mean changes in PANSS scores per time interval, per group:
| Change in PANSS Score |
||||
| Day | LUR40mg | LUR120mg | ZYP15mg | Placebo |
|
|
||||
| 4 | -5.1 | -5.4 | -6.1 | -4.8 |
| 7 | -10.4 | -8.7 | -11.0 | -7.2 |
| 14 | -15.4 | -13.8 | -16.3 | -11.6 |
| 21 | -18.5 | -20.9 |
-21.7 | -15.6 |
| 28 | -22.8 | -25.1 | -25.8 | -18.9 |
| 35 | -26.8 | -28.6 | -29.1 | -22.5 |
| 42 | -30.5 | -27.3 | -30.8 | -23.6 |
The article itself has the mean baseline PANSS scores. So applying the reported mean changes in the PANSS from this table to those baseline PANSS scores –
| Estimated Total Mean PANSS Scores |
||||
| Day | LUR40mg | LUR120mg | ZYP15mg | Placebo |
|
|
||||
| 0 | 96.6 | 97.9 | 96.3 | 95.8 |
| 4 | 91.5 | 92.5 | 90.2 | 91.0 |
| 7 | 86.2 | 89.2 | 85.3 | 88.6 |
| 14 | 81.2 | 84.1 | 80.0 | 84.2 |
| 21 | 78.1 | 77.0 |
74.6 | 80.2 |
| 28 | 73.8 | 72.8 | 70.5 | 76.9 |
| 35 | 69.8 | 69.3 | 67.2 | 73.3 |
| 42 | 66.1 | 70.6 | 65.5 | 72.2 |
– gives us a graph that is as close to the observed mean PANSS Total Scores as I can get without access to the raw data itself:
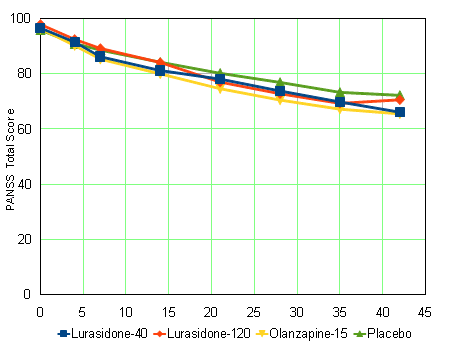
This American Journal of Psychiatry article is from Study D1050231, one of five considered by the FDA for in the approval process for Lurasidone [Latuda]. This next table summarizes the results of all [5] things considered [the D1050049 comparator was Haldol 10mg/day, the D1050231 comparator was Zyprexa 15mg/day] [synopsized from Drugs@FDA]:
| LS Mean Difference from Placebo | ||||||
| Study # | LUR20mg | LUR40mg | LUR80mg | LUR120mg | Comparator | |
|
|
||||||
| D1050006 US 2001 n=149 66% drop out |
BPRSd (LOCF) | -5.6 | -6.7 | |||
| BPRSd (MMRM) | -7.3 | -9.2 | ||||
| PANSS (LOCF) | -9.6 | -11.0 | ||||
|
|
||||||
| D1050049 US 2003 n=356 43% drop out |
BPRSd (LOCF) | -5.0 | -5.2 | -8.0 | -9.8 | -7.9 |
| PANSS (LOCF) | -7.1 | -7.2 | -13.6 | -16.0 | -12.3 | |
|
|
||||||
| D1050196 US 2004 n=180 45% drop out |
BPRSd (LOCF) | -4.7 | ||||
| PANSS (LOCF) | -8.6 | |||||
|
|
||||||
| D1050229 2008 n=496 34% drop out |
PANSS (MMRM) | -2.1 | -6.4 | -3.5 | ||
| PANSS (LOCF) | -2.7 | -6.1 | -3.5 | |||
| US Sites | +0.6 | -2.0 | +0.2 | |||
| Non-US Sites | -6.5 | -10.8 | -8.6 | |||
|
|
||||||
| D1050231 2009 n=475 38% drop out |
PANSS (MMRM) | -9.7 | -7.5 | -12.6 | ||
| PANSS (LOCF) | -7.9 | -4.8 | -11.4 | |||
| US Sites | -5.7 | -4.8 | -11.4 | |||
| Non-US Sites | -10.5 | -3.8 | -9.6 | |||
In general, this reviewer does not believe that D1050006 is an interpretable study for reasons outlined above. Study D1050229 is, in the opinion of this reviewer, marginal since only one of three lurasidone doses separated from placebo and this dose did not exhibit a robust signal in the US subgroup analysis. This reviewer considered studies D1050196 and D1050231 to be positive studies in support of the efficacy of lurasidone 40 mg [D1050231], 80 mg [D1050196] and 120 mg [D1050231] in the treatment of schizophrenia. However, since the efficacy of these doses of lurasidone has not been replicated in the other studies, this reviewer would recommend a complete response action at this time…
Based on the marginal efficacy data presented in this NDA and the pending availability of significant clinical trial data [both efficacy and safety] it seems premature to recommend a final action at this time. These recently completed clinical trials provide more efficacy and safety data for the 40 mg/day, 80 mg/day and a higher dose, 160 mg/day. These clinical trials are placebo controlled and both also include an active comparator. It would seem prudent to review the efficacy data from these recently completed Phase 3 trials before taking a final action since the data submitted in this NDA, in this reviewer’s opinion, do not support the efficacy of lurasidone in the treatment of schizophrenia…
1.2 Risk Benefit Assessment
Efficacy has not been established in this NDA submission. The safety profile is more similar to typical antipsychotics with significant akathisia, hyperprolactinemia, parkinsonian-adverse events and dystonias; many of which are dose-related. Lurasidone does not appear to have significant adverse impact on metabolic indices (glucose, lipids, weight, etc.). Lurasidone may be associated with potentially significant hypersensitivity reactions. A comprehensive risk:benefit assessment is premature at this time.
I failed in my goal of parsing out Quintiles’ specific contributions to the Lurasidone story. My guess is that they came into the picture at least for the last two studies based on the multinational sites and the speed to market [their specialty], but that’s just a guess. The CROs seem to make it their business to stay in the shadows. All Clinical Trials have simply "Sunovion Medical Director" as the Principle Investigator. And in this paper [Lurasidone in the treatment of schizophrenia: a randomized, double-blind, placebo and olanzapine controlled study] and the last one I looked at [Impact of Publicity Concerning Pediatric Suicidality Data on Physician Practice Patterns in the United States], Quintiles’ presence is minimally acknowledged. My bet is that Quintiles ran the whole show in both – again my guess.
What if the reviewer was Nemeroff or someone related to him?
A.L.
Hi 1BOM
This comment/link is referring to your post on 19 Oct 2011 “What’s the Fuss I & II”
Just a little more to add to the controversy of Prof P McGory and another mental health organizations he is associated with in Australia, “Orygen”
http://rc.oyh.org.au/ResearchCentreStructure/otherfunding
http://rc.oyh.org.au/ResearchCentreStructure/majorgrants
McGory also has associations with “Headspace”
http://www.1boringoldman.com hoped we’ve improved since the apogee of 2006… and yet
December 2011 British Journal of Psychiatry – puts Lunbeck advertisement into the scientific record
From “Highlights of the Issue†email:
Clinically important findings regarding use of risk assessment on wards and the ‘true’ effect of antidepressants
Two papers in the Journal this month examine common clinical interventions whose effectiveness has been questioned. Van de Sande et al (pp. 473–478) found that regular use of structured short-term risk assessment scales on acute psychiatric wards resulted in a reduction in the number of aggressive incidents, the number of patients who engaged in aggression and the time spent by in-patients in seclusion. Using a mixture model to identify patient subgroups, Thase et al (pp. 501–507) found evidence that the small average antidepressant–placebo difference found in many antidepressant trials may mask a much larger effect among those in the subgroup benefiting from treatment.
The latter refers to this paper:
Assessing the ‘true’ effect of active antidepressant therapy v. placebo in major depressive disorder: use of a mixture model
Michael E. Thase, Klaus G. Larsen, and Sidney H. Kennedy
The British Journal of Psychiatry 2011;199 501-507 Open access article
http://bjp.rcpsych.org/cgi/content/abstract/199/6/501
Which is an Lundbeck paper… written by:
Michael E. Thase, MD – an advisor/consultant for H. Lundbeck A/S
Klaus G. Larsen, Ph – an employee of H. Lundbeck A/S.
Sidney H. Kennedy, MD – received grant funding and consulting honoraria from H. Lundbeck A/S
“demonstrating†that…
Results
The MADRS scores improved by 15.9 points (95% CI 15.2–16.6) among patients who benefited from treatment. The proportion of patients who benefited from escitalopram and not from placebo treatment was 19.5%, corresponding to a number needed to treat of 5.
Conclusions
This model gave a considerably better fit to the data than the analysis of covariance model in which all patients were assumed to benefit from treatment. The small average antidepressant–placebo difference obscures a much larger effect in a clinically meaningful subgroup of patients.
Is there ANY problem with this material being “on the scientific record�