-
To develop practice systems that facilitate recognition and care of depression;
-
To develop educational programs and tools for primary care that enhance patient care;
-
To evaluate the impact of these programs, tools and models; and
-
To disseminate these ideas and materials to primary care clinicians, medical groups, specialty societies, health plans, and other organizations committed to high quality depression care.
Prepared by Michael B. First, M.D., DSM Consultant to the American Psychiatric Institute for Research and Education [APIRE], a subsidiary of the American Psychiatric Association.A diagnosis-related research planning conference focusing on dimensional approaches in diagnostic classification was held at the Natcher Conference Center on the NIH campus in Bethesda, Maryland on July 27th and 28th, 2006. The conference was the seventh in a series of 12 conferences on "The Future of Psychiatric Diagnosis: Refining the Research Agenda" convened by the American Psychiatric Institute for Research and Education [APIRE] in collaboration with the WHO and NIH, with funding from NIH…
John Helzer, MD [Burlington, VT] opened the conference with a description of its rationale and goals These were:
He stressed that the six diagnostic areas scheduled for formal presentation – i.e., substance use disorders, mood disorders, psychotic disorders, anxiety disorders, childhood disorders, and personality disorders – were illustrative examples and that it was not the intention of the conference to confine discussion to these areas. Dr. Helzer noted that although DSM-III was a revolutionary paradigmatic shift, the diagnostic progress it fostered revealed the inadequacies of a strictly categorical diagnostic model. He then offered four recommendations for DSM-V:
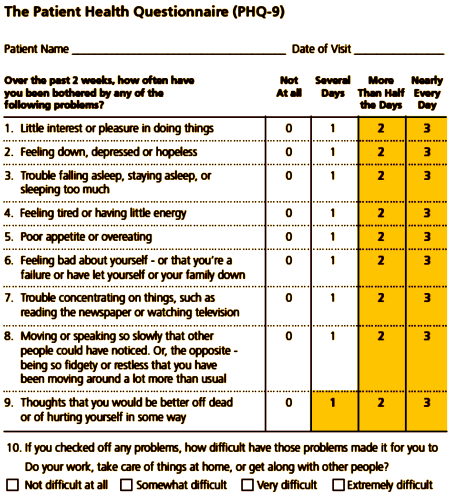
The mood disorders breakout group proposed adding dimensionality to major depressive disorder by exploring the implications of adopting the PHQ-9, a nine-item self-report scale with a 0-27 range of scores. Used as a severity measure, suggested cutpoints include 5-9 for mild, 10-14 for moderate, 15-19 for moderately severe, and 20 or greater for severe, with "significantly improved" defined as a 5-point drop after 6 weeks, "responded to treatment" as a 50% drop in score, and "remission of depression" as a score of less than 5. Such an approach has clinical utility in that it can identify probable cases, it identifies cases at risk who need referral, it confirmed current treatment in 60% of cases, it informed a change of treatment in 40% of cases, and in >90% of visits, clinicians rated it as useful. It has research utility in that it sets benchmarks for improvement, recovery, and remission and permits clinical audits of practice. The scale still needs validation in different settings and with different types of patients [e.g., high risk patients with comorbid conditions].
1 I occasionally feel indecisive or find that my attention wanders.
2 Most of the time, I struggle to focus my attention or to make decisions.
3 I cannot concentrate well enough to read or cannot make even minor decisions.
1 I am more self-blaming than usual.
2 I largely believe that I cause problems for others.
3 I think almost constantly about major and minor defects in myself.
The provenance of the PHQ-9 could bear some scrutiny. It is a creation of Robert Spitzer, architect of DSM-III. So, it should surprise nobody that the 9 items in the PHQ-9 mirror the 9 symptoms in the DSM-III criteria for major depressive episode. It was embedded in a larger instrument (PRIME-MD) that Pfizer underwrote. Here is the boilerplate from the Macarthur Foundation (http://www.depression-primarycare.org/clinicians/toolkits/materials/forms/phq9/) :
The PHQ-9 is adapted from PRIMEMDTODAY, developed by Drs. Robert L. Spitzer, Janet B.W. Williams, Kurt Kroenke, and colleagues, with an educational grant from Pfizer Inc. For research information, contact Dr Spitzer at rls8@columbia.edu. The names PRIME-MD® and PRIMEMDTODAY™ are trademarks of Pfizer Inc.
PHQ9 Copyright © Pfizer Inc. All rights reserved. Reproduced with permission of Pfizer, Inc. PRIME-MD ® is a trademark of Pfizer Inc.
These 9 items then were formatted into a Zung scale-like format that treats frequency of a symptom as equivalent to its severity (not!). It looks like the Macarthur Foundation and its money contributed no added value. One is reminded of the saying that a camel is a horse designed by a committee. It seems that DSM-5 bids fair to be a cavalcade of camels.
Boy, I sure missed that page! What are they trying to do? Buy Spitzer off? The Cavalcade of Camels would make a great book title for the DSM-5…
It’s trolling for patients, plain and simple. By asking how often a person has felt even slightly lousy over a relatively short time period, you allow people to be diagnose as depressed who might simply be responding to many of life’s ups and downs. A person whose company has just been bought and is waiting if they will have a job, someone in arbitration with the IRS, a million different things could cause someone to have those symptoms.
FYI
“Despite the potential for pharmacogenomics to improve patient care, we have yet to see a pharmacotherapeutic paradigm shift in which information on a patient’s genetic makeup is used with other patient-specific factors to guide therapy decisions.—
“Mrazek predicted that it won’t be too long before diagnostic and treatment guidelines for psychiatric disorders recommend pharmacogenomics testing as part of the initial workup, to protect patients from adverse effects and to decrease the time and cost in identifying the right medicines.”
“In their JAMA article, Mrazek and coauthor Lerman, Professor of Psychology in the Department of Psychiatry at the University of Pennsylvania, urged that pragmatic clinical trials (PCTs) be considered, rather than just exclusive reliance on randomized controlled trials.
“Once initial efficacy has been documented, a PCT could be part of the validation pathway for implementation of pharmacogenomic tests,†they wrote.”
Psychiatric Pharmacogenomics
By Arline Kaplan | December 12, 2011
http://www.psychiatrictimes.com/display/article/10168/2004602
Uh, oh! Does he mean a pragmatic clinical trial like STAR*D? LOL.
In pursuit of the grand obsession, NIMH has poured millions of dollars down the drain with pharmacogenetic add-on studies to crappy trials like STAR*D, where the quality of the clinical data is next to worthless.