Record Breaking $327 Million Verdict Upheld in Janssen Case and Request for New Trial Denied
Dec 21, 2011The jury verdict in the case of State of South Carolina versus Ortho-McNeil-Janssen Pharmaceuticals and Johnson & Johnson, Inc. has been upheld and requests for a new trial denied, affirming groundbreaking $327 million in civil penalties against the manufacturers of the drug Risperdal. Circuit Court Judge Roger Couch announced the rulings on December 20 through two written orders. One order denies the defendant’s motion for judgment notwithstanding the verdict or, in the alternative, for a new trial; the second order denies the defendant’s motion to alter or amend the judgment and/or for a new trial. John B. White, Jr. and Donald C. Coggins, Jr. of Harrison, White, Smith & Coggins, P.C., a Spartanburg-based law firm, along with John Simmons of the Simmons Law Firm, a Columbia-based law firm, and Bailey Perrin Bailey, a Texas based law firm represented South Carolina in the case.
"We are obviously very pleased with Judge Couch’s decision and his careful consideration of this matter," stated John B. White, Jr. one of the attorneys representing the state in the case. "The verdict handed down by the jury is just and speaks the truth. The damages awarded further substantiated the level of deception Janssen used in business practices in our state. Once again, we have sent a clear message to drug companies that deceptive business practices will not be tolerated in South Carolina"…
J&J Is Ordered to Pay $327 Million on Risperdal Deceptive-Marketing Claims
Bloomberg
By Jef Feeley and Steven Church
June 3, 2011
A Johnson & Johnson unit must pay more than $327 million in penalties for deceptively marketing the antipsychotic drug Risperdal as safer and better than competing medicines, a South Carolina judge ruled. J&J’s Ortho-McNeil-Janssen Pharmaceuticals unit repeatedly violated South Carolina’s consumer-protection laws by sending a 2003 letter to doctors touting Risperdal as superior to rival drugs and including deceptive information in the product’s warning label, Judge Roger Couch in Spartanburg, South Carolina, concluded.“We don’t believe that the dissemination of an FDA approved package insert constitutes a violation of the South Carolina Trade Practices Act,” Kara Russell, a spokeswoman for Janssen said in an e-mailed statement. “We do not believe the ruling can be upheld on appeal.”South Carolina’s lawyers, who originally sued the J&J unit in 2007 for making misleading claims about Risperdal, sought billions of dollars in penalties over the marketing.
Risperdal’s global sales peaked at $4.5 billion in 2007 and declined after the company lost patent protection. The drug generated $3.4 billion in sales in 2008, or 5.4 percent of J&J’s total revenue, according to company filings. Sales of the drug fell to $527 million last year, according to a January earnings report…
The case is the third of about 10 state lawsuits to be considered by jurors over J&J’s Risperdal marketing campaigns. In June, J&J won dismissal of Pennsylvania’s suit alleging the company hid the drug’s diabetes risk and tricked regulators into paying millions more than they should have for the medicine. A Louisiana jury in October ordered the drugmaker to pay $257.7 million in damages to that state for making misleading claims about Risperdal’s safety. A judge later added $73 million in legal fees to the award.
A West Virginia judge in a 2009 non-jury trial awarded $3.95 million, finding the company misled doctors about the risks and benefits of Risperdal. The state dropped its Risperdal claim after J&J won an appeal, company officials said in February…
While it’s good news that Johnson&Johnson is being punished for some pretty outrageous behavior, there remains the issue of "the cost of doing business" – the fact that these large settlements, even with their legal fees, are a drop in the bucket compared to their profits:
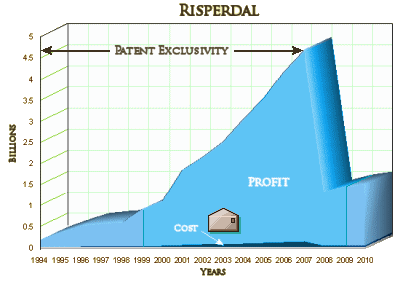
The Whistle-Blower suit coming up in Texas next month is a different kind of suit from the ones tried so far. The sins are greater and the stakes are higher. The allegation is that J&J and subsidiaries "fixed" the Tri-University Guidelines, funded and influenced the Texas Medical Algorithm Project that defrauded the state’s Medicaid system, then funded Texas officials to carry the program out to about a third of our other states. This was much more than simply omitting negative information thereby misrepresenting their product. It was an active campaign involving paying off officials to create a system that preferentially used their drugs at great expense with no improvement in efficacy. More than cover-up, this was an outright scam.

Right on all counts Mickey.
I honestly hope that this latest “victory” (you’re right that it will just be seen as a cost-of-business issue for J&J) will be seen in hindsight as a watershed moment that makes it that much easier for courts to really sharpen the knives and start cutting deep into pharma’s pockets. I’m talking multi-billion dollar fines/settlements that really put the hurt on the buttoned-down creeps in the board-rooms.
And gosh who knows? Maybe sometime while we’re still living CRIMINAL charges for those involved? I can always dream can’t I? Indeed it will be interesting to see how the TMAP trial goes.
PS: Great to see my favorite graph making an encore! That Risperdal profit margin graph will forever be my favorite eye-bugger. Nothing puts it more in perspective faster than that sucker.
Fines.
Always fines.
And never prison time.
I agree, the fines are a drop-in-the-bucket for these pharma companies.
Of course, to push for some prison time, we will need a real Justice Department and a real U.S. Attorney General…
That’s another subject altogether (another post, another blog, etc)…
I appreciate the clock on your website, with a countdown to the days left on Obama’s watch (and the head of the justice department).
Duane
PTSD treatment for Veterans found ineffective.
Risperdal can cause diabetes.
I took Lilly Olanzapine a powerful schizophrenic drug (same as Risperdal) for 4 years it was prescribed to me off-label for post traumatic stress disorder was ineffective costly and gave me diabetes.
*FIVE at FIVE*
The Zyprexa antipsychotic drug,whose side effects can include weight gain and diabetes, was sold for “children in foster care, people who have trouble sleeping, elderly in nursing homes.
*Five at Five* was the Zyprexa sales rep slogan, meaning *5mg dispensed at 5pm would keep patients quiet*.
— Daniel Haszard Zyprexa victim activist