I have repeatedly teased about Dr. Trivedi’s difficulty finishing tasks. His IMPACTS study was never completed. In STAR*D, they never published their primary efficacy results. CO-MED came in way late and was decidedly negative. TMAP landed in a courtroom. But in EMBARC, his new genetics study, he didn’t even finish his web-site.
Why, pray tell, was I looking at the
EMBARC web site? I had something rolling around I wanted to say about the NIMH study. This is the one where he’s looking for
biosignatures to predict the response to antidepressants. Here’s what I thought they were going to do: Put people on either Citalopram or Bupropion measuring a gajillion potential biomarkers, then crossover and put the non-responders on the other drug – all the while collecting the gajillion biomarkers looking for some thing [or things] that would predict response. That’s what I remembered. But when I started to look for the details…
NIMH RePORTER: We propose a comparative effectiveness trial of three mechanistically distinct treatments for MDD [citalopram, bupropion, and cognitive behavioral therapy] in which we will assess a comprehensive array of carefully selected clinical [i.e. anxious depression, early life trauma, & gender] and biological [i.e. genetic, neuroimaging, serum, epigenetic & qEEG] moderators and mediators of outcome. Using innovative statistical approaches the identified moderators and mediators will then be used to develop a differential depression treatment response index [DTRI]. The proposed study is a randomized two-stage trial [Stagel:12 wks; Stage2: 12 wks] design with 675 MDD patients [with a history of one adequate trial of an SSRI except citalopram] assigned to one of three treatment conditions [n=225 each]. This two stage approach is similar to a Sequential Multiple Assignment Randomized Trial [SMART] design. This study will examine multiple carefully selected clinical and biological markers, using both existing state of-the-art technologies as well as pioneering, innovative approaches. Evaluation of the usefulness of these markers in a trial with three different treatments will assist in generating a depression treatment response index [DTRI]. The DTRI will help clinicians match treatments to patients with MDD, resulting in timely selection of treatments best suited for individual patients. Results from this study could significantly improve the treatment of patients with MDD.&
clinicaltrials.gov: The current study is designed to identify biomarkers for the prediction of differential treatment outcomes between the SSRI antidepressant citalopram [CIT] and placebo [PBO] in a randomized trial for patients with MDD. In addition, a second stage will collect data to explore moderators and mediators of treatment outcomes between pharmacologically distinct active treatment arms: citalopram [CIT], a serotonergic antidepressant or bupropion [BUP], a nonserotonergic antidepressant… In the first stage, patients will receive an 8−week course of treatment in one of the two study arms. As part of the Sequential Multiple Assignment Randomized Trial [SMART] design patients that have not achieved response at the end of 8 weeks to their stage one treatment, defined by < 50% improvement on the Clinical Global Improvement scale [CGI], will be switched to Stage 2 treatment [8 weeks]. Patients who have achieved satisfactory response [>= 50% improvement on the CGI] will be continued on treatment for an additional 8 weeks.
After reading those two descriptions, I haven’t got any idea what they’re going to do. But I’ll make my comment anyway, just in the offhand case that when the study becomes clear, it fits. I think the hypothesis for the study is that there is some genetic factor that has to do with whether or not you respond to one antidepressant or another. I can’t find solid evidence for such a notion, I just know it’s a widely held hypothesis. So they’re going to study a group of depressed people hypothesizing that the group contains Citalopram Responders and Bupropion Responders which they’re going to identify using biosignatures. My comment was going to be "Don”t bother. You’ve already done the study." Remember CO-MED?
Combining Medications to Enhance Depression Outcomes (CO-MED): Acute and Long-Term Outcomes of a Single-Blind Randomized Study
American Journal of Psychiatry
by A. John Rush, M.D., Madhukar H. Trivedi, M.D., Jonathan W. Stewart, M.D., Andrew A. Nierenberg, M.D., Maurizio Fava, M.D., Benji T. Kurian, M.D., Diane Warden, Ph.D., David W. Morris, Ph.D., James F. Luther, M.A., Mustafa M. Husain, M.D., Ian A. Cook, M.D., Richard C. Shelton, M.D., Ira M. Lesser, M.D., Susan G. Kornstein, M.D., and Stephen R. Wisniewski, Ph.D.
May 2, 2011
Objective: Two antidepressant medication combinations were compared with selective serotonin reuptake inhibitor monotherapy to determine whether either combination produced a higher remission rate in first-step acute-phase (12 weeks) and long-term (7 months) treatment.
Method: The single-blind, prospective, randomized trial enrolled 665 outpatients at six primary and nine psychiatric care sites. Participants had at least moderately severe nonpsychotic chronic and/or recurrent major depressive disorder. Escitalopram (up to 20 mg/day) plus placebo, sustained-release bupropion (upto 400 mg/day) plus escitalopram (up to 20 mg/day), or extended-release venlafaxine (up to 300 mg/day) plus mirtazapine (up to 45 mg/day) was delivered (1:1:1 ratio) by using measurement-based care.The primary outcome was remission, defined as ratings of less than 8 and less than 6 on the last two consecutive applications of the 16-item Quick Inventory of Depressive Symptomatology-Self-Report. Secondary outcomes included side effect burden, adverse events, quality of life, functioning, and attrition.
Results: Remission and response rates and most secondary outcomes were not different among treatment groups at 12 weeks. The remission rates were 38.8% for escitalopram-placebo, 38.9% for bupropion-escitalopram, and 37.7% for venlafaxine-mirtazapine, and the response rates were 51.6%–52.4%. The mean number of worsening adverse events was higher for venlafaxine-mirtazapine (5.7) than for escitalopram-placebo (4.7). At 7 months, remission rates (41.8%–46.6%), response rates (57.4%–59.4%), and most secondary outcomes were not significantly different.
Conclusions: Neither medication combination outperformed monotherapy. The combination of extended-release venlafaxine plus mirtazapine may have a greater risk of adverse events.
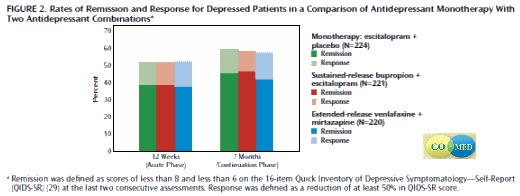
I’m assuming that Citalopram [Celexa] and Escitalopram [Lexapro] are bioequivalent drugs. So, if there were two populations [Citalopram Responders and Bupropion Responders], in CO-MED there would’ve been a greater response to Escitalopram+Bupropion than to Escitalopram+Placebo. It didn’t happen.
Thus far, attempts to squeeze more out of the antidepressants than they offer up front haven’t been very successful. I doubt that either the
EMBARC study or
iSPOT will be any different. I suppose if you think the only thing you have to offer is drugs, hope springs eternal…


Typical Trivedi, wasting precious taxpayer dollars on poorly run clinical trials.
Yes, he’s the master at that…