Targacept: What’s Left After Latest TC-5214 Disappointment?
Seeking Alpha
by Bryce Istvan
December 20, 2011
Today AstraZeneca and Targacept announced the results from their second of four Phase 3 trials for TC-5214, a depression drug. The results failed to meet the primary endpoint based on change in the Montgomery-Asberg Depression Rating Scale total score after eight weeks of adjunct treatment with TC-5214 as compared to placebo. This is the second failed Phase 3 trial for TC-5214. On November 8 the company announced the failure of the first trial and shares were cut in half. There are two more trials ongoing with results expected in the first half of 2012; the ongoing trials utilize a fixed dosing regimen rather than the variable doses used in the first two.Targacept and AstraZeneca have a research agreement wherein AstraZeneca is generally responsible for the funding and management of clinical trials and commercialization. AstraZeneca has announced it is continuing the remaining trials, but will review their regulatory filing targets once all data is available. Previously it expected to file a New Drug Application in the second half of 2012. Additionally, they are writing down the value of their TC-5214 assets by 50% to $96.5 million “based on the lower probability of success for the remaining TC-5214 studies.” Based on two failed trials it is increasingly unlikely that TC-5214 will ever see an FDA approval. However, Targacept does have other drugs coming down the pipeline.
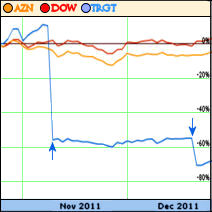 With all the recent moaning about the pharmaceutical industry fleeing CNS drug development, the bottom line is that they don’t have much to work with these days. There are a finite number of neurotramsmitter receptor systems to tweak, and most of the major ones are already heavily tweaked. A recent candidate, Mecamylamine, a nonselective and noncompetitive antagonist of the nicotinic acetylcholine receptors, seems to be failing in clinical trials as we speak [as well as on the stock market]. AstraZeneca paid Targacept $200 M in a licensing agreement in 2009 and just took a $380 M write down in value after this [and other] failed clinical trials. I’m incapable of comprehending numbers of this magnitude, but it seems like a lot of money.
With all the recent moaning about the pharmaceutical industry fleeing CNS drug development, the bottom line is that they don’t have much to work with these days. There are a finite number of neurotramsmitter receptor systems to tweak, and most of the major ones are already heavily tweaked. A recent candidate, Mecamylamine, a nonselective and noncompetitive antagonist of the nicotinic acetylcholine receptors, seems to be failing in clinical trials as we speak [as well as on the stock market]. AstraZeneca paid Targacept $200 M in a licensing agreement in 2009 and just took a $380 M write down in value after this [and other] failed clinical trials. I’m incapable of comprehending numbers of this magnitude, but it seems like a lot of money.
If these psychiatric research groups were structural engineers, the sales presentation would go like this –
“Why build one ill-conceived, poorly-designed, unsound and dangerous toll bridge, when you can build two… for twice the cost, and ten times the return on investment?”
Psychiatric research… the gift that keeps on giving…. world-wide.
Duane
Mickey,
There are 39,000 members of the APA.
39,000.
A few psychiatrists are bloggers.
A few.
I don’t know if you’re aware of the fact that you are almost all-alone out there, when it comes to presenting the facts, and really taking on the status quo (the failed paradigm of “care”).
IMO, you’re not “one boring old man”… you’re “one gutsy retired doc.”
That’s my call.
Thank you, for all you’re doing!
Duane
This is an example of what we could call rabbit in a hat ‘pretend’ clinical science. The narrative goes like this: We announce a novel candidate antidepressant drug. We pretend to have a scientific basis for testing this compound, based on a pretend animal model of depression and a pretend neurochemical theory. We do a lucrative licensing deal with a major pharmaceutical corporation. Then, since it is now other people’s money at risk, we go full bore with enrolling pretend patients by the hundreds at dozens of testing sites globally. These pretend patients carry the pretend diagnosis of major depression, which itself is a fictive entity. We bolster the illusion of science by surrounding the project with the trappings of science in the form of a multinational CRO.
Like Targacept, another corporation that has played this kind of game to a fare-thee-well is Corcept Therapeutics, the company founded by Alan Schatzberg. At last count they have gone through more than $200 million of invested capital with nothing to show for it in terms of their lead drug for psychotic depression. Along the way they set some kind of a record for placebo response rates north of 80% in that condition, which Schatzberg himself acknowledges should have a low rate of response to placebo: so much for quality control in Corcept’s Phase III trials. Worse, though their candidate drug supposedly has a neurochemical theory behind it (blocking cortisol receptors in depressed patients with psychotic symptoms caused by high cortisol levels) they have not reported on high cortisol to validate the subjects enrolled in their Phase III trials. Thus does rigorous clinical science suffer in the service of commercial goals.
Dr. Carroll,
You’re eloquent on this one! Thanks for mentioning the “pretend animal model of depression…” I just didn’t have the heart to go into those poor little mice swimming for their lives…
Duane,
Thanks for the nice words.
I was somewhat surprised by the dismal results of the Phase 3 studies of TC-5214, considering the encouraging Phase 2b trial results…
“Dr. Madhukar H. Trivedi, one of the principal investigators for the [Phase 2b] trial, said that the magnitude and consistency of the effect of TC-5214 in this trial could represent a breakthrough for patients with depression.
“It is particularly compelling that the superiority of TC-5214 as augmentation to citalopram over citalopram alone was first seen after only two weeks and grew steadily over the trial’s last six weeks, culminating in remission for twice as many subjects in the TC-5214 group,’ Trivedi said (Daniel, Fran. “Targacept stock jumps after news from drug trial.†Winston-Salem Journal 16 July 2009.)â€
Fortunately, I did not ‘buy’ Trivedi’s story.
Nancy Wilson,
You are a certified super sleuth! I had no idea that that the ubiquitous Dr. Trivedi was involved with that Phase II Trial [Targacept stock jumps after news from drug trial]. As is usual with him, there’s something screwy. In the Clinical Trial, Trivedi is listed as Principle Investigator for the UT Southwestern site, but not the whole study. Then, on 11/ 09/2008, he’s replaced by someone else, [before this article mentioned above] and doesn’t appear in the Clinical Trials Archive [whatever that means]:
Good sleuthing…
Barney Carroll’s comments are spot on. But it’s incomprehensible why a company like AstraZeneca would not do real science rather than pseudoscience, since only real science will lead to real clinical pay offs. At a minimum, one would try to sort the “depressives” into more homogeneous groups by first administering the DST. It’s also not understandable why Schatzberg, in search of an agent to treat psychotic depression, didn’t screen the candidates for a trial with the DST. The neglect of pharmaco-EEG in assessing these agents is equally bewildering. It’s as though the 1960s and 70s had somehow not happened.
To expand a little on Dr. Shorter’s comment, Corcept has pissed away over $200 million and they still have not straightforwardly answered the original question, which is does their drug benefit patients with psychotic depression that is accompanied by high cortisol levels? Corcept strikes me as a corporation that suffers from Ganser syndrome, the syndrome of approximate answers. Everything is Vorbeireden, off topic, beside the point, never getting to the real issue, never incisively testing the main question, all the while engaging in hand waving and special pleading.