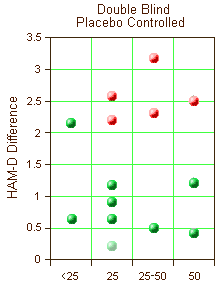
 The chart on the right is a simplification of a large table in a recently published paper. The ordinate is the mean difference of the HAM-D scores between drug and placebo [except the ghost point which is a MADRS difference – included to show non-significance]. The abscissa is the daily drug dose. Each point is a different clinical trial/dose pair from nine Double Bind, Placebo Controlled clinical trials. The significant studies [p<0.05] are in red. Those in green failed to separate from placebo. 5/14 significant. 9/14 not significant. All but one study <3.0 points Difference on the HAM-D. No dose response curve.
The chart on the right is a simplification of a large table in a recently published paper. The ordinate is the mean difference of the HAM-D scores between drug and placebo [except the ghost point which is a MADRS difference – included to show non-significance]. The abscissa is the daily drug dose. Each point is a different clinical trial/dose pair from nine Double Bind, Placebo Controlled clinical trials. The significant studies [p<0.05] are in red. Those in green failed to separate from placebo. 5/14 significant. 9/14 not significant. All but one study <3.0 points Difference on the HAM-D. No dose response curve.
Five of the nine Double Bind, Placebo Controlled clinical trials reviewed had an Active Comparator included [Paroxetine (3) and Fluoxetine (2)]. Again, the significant studies [p<0.05] are in red and those in green failed to separate from placebo. The active comparators have a tan shaded background.
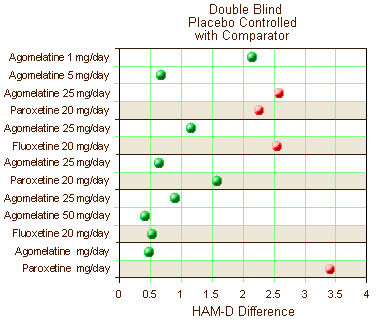
Certainly no improvement there. Only one trial showed clinical significance for the drug whereas 3/5 Active Comparators separated from Placebo.They also had six clinical trials where they’d studied Agomelantine "head to head" with Active Comparators:
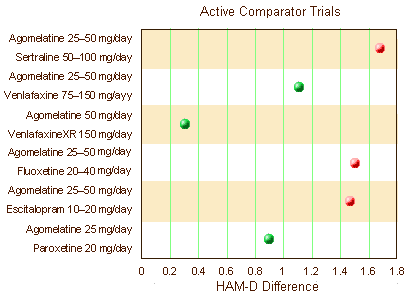
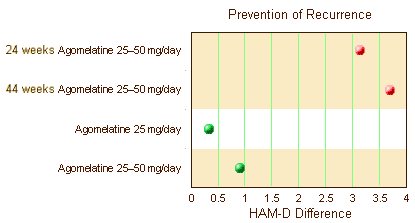
Before getting too excited that they finally broke even on statistical significance, look at the units on the abscissa. The differences were under two points on the HAM-D scale [you could probably do that with few good nights’ sleep]. They also reviewed three Prevention of Recurrence Clinical Trials. One was significant. The other two weren’t [duration unspecified]:
Novel melatonin-based therapies:
potential advances in the treatment of major depression
by Ian B Hickie and Naomi L Rogers
Lancet 2011 378: 621–31.
[full text online]
Major depression is one of the leading causes of premature death and disability. Although available drugs are effective, they also have substantial limitations. Recent advances in our understanding of the fundamental links between chronobiology and major mood disorders, as well as the development of new drugs that target the circadian system, have led to a renewed focus on this area. In this review, we summarise the associations between disrupted chronobiology and major depression and outline new antidepressant treatment strategies that target the circadian system. In particular, we highlight agomelatine, a melatonin-receptor agonist and selective serotonergic receptor subtype (ie, 5-HT2C) antagonist that has chronobiotic, antidepressant, and anxiolytic effects. In the short-term, agomelatine has similar antidepressant efficacy to venlafaxine, fluoxetine, and sertraline and, in the longer term, fewer patients on agomelatine relapse (23·9%) than do those receiving placebo (50·0%). Patients with depression treated with agomelatine report improved sleep quality and reduced waking after sleep onset. As agomelatine does not raise serotonin levels, it has less potential for the common gastrointestinal, sexual, or metabolic side-effects that characterise many other antidepressant compounds.
Conclusions
Melatonin analogues provide a new and efficacious mechanism for producing notable phase shifts in human beings. Although these drugs have been mainly studied for sleep disorders, they also have the potential to be used as primary or adjunctive drugs across a wider range of neuropsychiatric disorders characterised by persistent circadian disturbance. Importantly, only agomelatine (which also binds 5-HT2C receptors) has been reported to have clinically significant antidepressant effects. Because of its favourable adverse effect and safety profile, and the potential to help to restore circadian function between depressive episodes, this drug might occupy a unique place in the management of some patients with severe depression and other major mood disorders.
Contributors
Both authors participated in the conception and writing of this article and have seen and approved the final version.
Conflicts of interest
IBH was previously chief executive officer and clinical adviser of beyondblue, an Australian National Depression Initiative. He has led projects for health professionals and the community supported by governmental, community agency, and drug industry partners (Wyeth, Eli Lily, Servier, Pfizer, AstraZeneca) for the identification and management of depression and anxiety. He has served on advisory boards convened by the drug industry in relation to specific antidepressants, including nefazodone, duloxetine, and desvenlafaxine, and has participated in a multicentre clinical trial of agomelatine effects on sleep architecture in depression. IBH is also supported by a National Health and Medical Research Council Australian Medical Research Fellowship. He is a participant in a family-practice-based audit of sleep disturbance and major depression, supported by Servier, the manufacturers of agomelatine. NLR has received grant support from Vanda Pharmaceuticals, Servier, Pfizer, and Cephalon, and has received honoraria for lectures from Pfizer, CSL Biotherapies, and Servier. She has previously received research funding from Vanda Pharmaceuticals, manufacturers of tasimelteon. She has also received an unrestricted educational grant from Servier. Research studies done by IBH and NLR are mainly funded by NHMRC project and program grants.
The US Novartis trial of sub lingual agomelatine was terminated. I wasn’t informed, but I was a subject in that study and had a DILI 1 week after drug d/c (for worsening symptoms). I requested that it be reported, but I can’t find any evidence that it was. IRB/CRO/Novartis not interested. Link to the ClinicalTrials.gov study info at my name.
I don’t think there is any ability at this juncture for Novartis to submit anything to the FDA for approval in the US.
If you get a moment, would you please check to see if my recently submitted comments have landed in your spam file? Thanks-
But here in Oz I never realised that IBH had that kind of profile with the pharmaceuticals…his public profile through Beyond Blue is so high that no one would publicly criticise what he says – whatever might be said behind closed doors. Thanks!
What’s also interesting is that the second author (Naomi Rogers) works in the School of Management and Marketing. I think it’s doubtful that she’s a clinician or a neuroscientist. So, why is a marketing consultant writing a paper on Agomelatine?
The acknowledgements section gives credit to Bradley Whitwell, who is a graphic artist and undoubtedly did some of the graphics. Tracey Davenport and Georgine Luscombe work for Academic Research and Statistical Consulting (ARSC) and their presence as authors on a wide range of papers and reports with very varied subjects points to roles akin to contract research, or contract authors. I can’t find a website for ARSC, however, and they have affiliations to academic departments in Sydney.
The fact that the main authors only saw and approved the final manuscript (rather than wrote it) is alarming and is a bit of a giveaway, in my view.
ajackson – you should be more careful with your google searches. Brad Whitwell is a research fellow with the Chronobiology group at the Brain & Mind Research Institute (same place as Ian Hickie). Naomi Rogers is not a marketing consultant. She was the head of the Chronobiology lab at Brain & Mind before moving to CUQ. This explains the odd sounding affiliation- they’ve just plugged her into the existing bureacratic structure.