Double-Blind Comparison of First- and Second-Generation Antipsychotics in Early-Onset Schizophrenia and Schizo-affective Disorder: Findings From the Treatment of Early-Onset Schizophrenia Spectrum Disorders (TEOSS) Study
by Linmarie Sikich; Jean A. Frazier; Jon McClellan; Robert L. Findling; Benedetto Vitiello; Louise Ritz; Denisse Ambler; Madeline Puglia; Ann E. Maloney; Emily Michael; Sandra De Jong; Karen Slifka; Nancy Noyes; Stefanie Hlastala; Leslie Pierson; Nora K. McNamara; Denise Delporto-Bedoya; Robert Anderson; Robert M. Hamer; and Jeffrey A. Lieberman
American Journal of Psychiatry. 2008 165:1420-1431.
[full text online]
Objective: Atypical [second-generation] antipsychotics are considered standard treatment for children and adolescents with early-onset schizophrenia and schizoaffective disorder. However, the superiority of second-generation antipsychotics over first-generation antipsychotics has not been demonstrated. This study compared the efficacy and safety of two second-generation antipsychotics (olanzapine and risperidone) with a first-generation antipsychotic (molindone) in the treatment of early-onset schizophrenia and schizoaffective disorder.
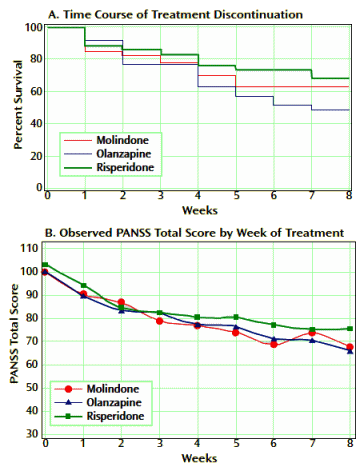
Method: This double-blind multisite trial randomly assigned pediatric patients with early-onset schizophrenia and schizoaffective disorder to treatment with either olanzapine (2.5—20 mg/day), risperidone (0.5—6 mg/day), or molindone [10—140 mg/day, plus 1 mg/day of benztropine] for 8 weeks. The primary outcome was response to treatment, defined as a Clinical Global Impression (CGI) improvement score of 1 or 2 and ≥20% reduction in Positive and Negative Syndrome Scale [PANSS] total score after 8 weeks of treatment.
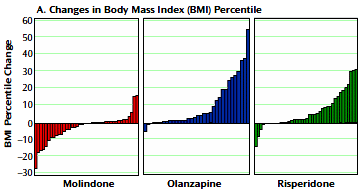
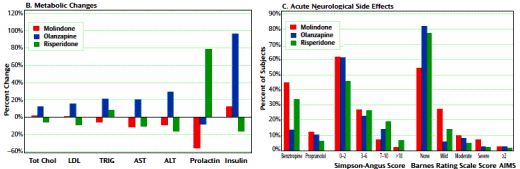
Results: In total, 119 youth were randomly assigned to treatment. Of these subjects, 116 received at least one dose of treatment and thus were available for analysis. No significant differences were found among treatment groups in response rates (molindone: 50%; olanzapine: 34%; risperidone: 46%) or magnitude of symptom reduction. Olanzapine and risperidone were associated with significantly greater weight gain. Olanzapine showed the greatest risk of weight gain and significant increases in fasting cholesterol, low density lipoprotein, insulin, and liver transaminase levels. Molindone led to more self-reports of akathisia.
Conclusions: Risperidone and olanzapine did not demonstrate superior efficacy over molindone for treating early-onset schizophrenia and schizoaffective disorder. Adverse effects were frequent but differed among medications. The results question the nearly exclusive use of second-generation antipsychotics to treat early-onset schizophrenia and schizoaffective disorder. The safety findings related to weight gain and metabolic problems raise important public health concerns, given the widespread use of second-generation antipsychotics in youth for nonpsychotic disorders.
Double-blind maintenance safety and effectiveness findings from the Treatment of Early-Onset Schizophrenia Spectrum [TEOSS] study
by Findling RL, Johnson JL, McClellan J, Frazier JA, Vitiello B, Hamer RM, Lieberman JA, Ritz L, McNamara NK, Lingler J, Hlastala S, Pierson L, Puglia M, Maloney AE, Kaufman EM, Noyes N, and Sikich L.
Journal of the American Academy of Child and Adolescent Psychiatry. 2010 49(6):583-94.
[full text online]
OBJECTIVE: To examine the long-term safety and efficacy of three antipsychotics in early-onset schizophrenia spectrum disorders.
METHOD: Patients [8 to 19 years old] who had improved during an 8-week, randomized, double-blind acute trial of olanzapine, risperidone, or molindone [plus benztropine] were eligible to continue on the same medication for up to 44 additional weeks under double-blind conditions. Adjunctive medications were allowed according to defined algorithms. Standardized symptom, safety, and functional assessments were conducted every 4 weeks.
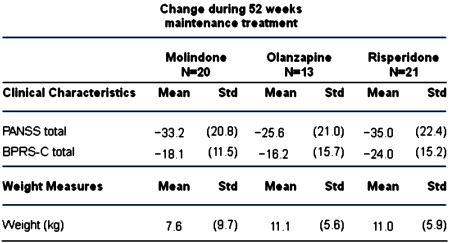
RESULTS: Of the 116 youths randomized in the acute trial, 54 entered maintenance treatment [molindone, n = 20; olanzapine, n = 13; risperidone, n = 21]. Fourteen [26%] completed 44 weeks of treatment. Adverse effects [n = 15], inadequate efficacy [n = 14], or study nonadherence [n = 8] were the most common reasons for discontinuation. The three treatment arms did not significantly differ in symptom decrease or time to discontinuation. Akathisia was more common with molindone and elevated prolactin concentrations more common with risperidone. Although weight gain and metabolic adverse events had occurred more often with olanzapine and risperidone during the acute trial, no significant between-drug differences emerged in most of these parameters during maintenance treatment.
CONCLUSIONS: Only 12% of youths with early-onset schizophrenia spectrum disorders continued on their originally randomized treatment at 52 weeks. No agent demonstrated superior efficacy, and all were associated with side effects, including weight gain. Improved treatments are needed for early-onset schizophrenia spectrum disorders.
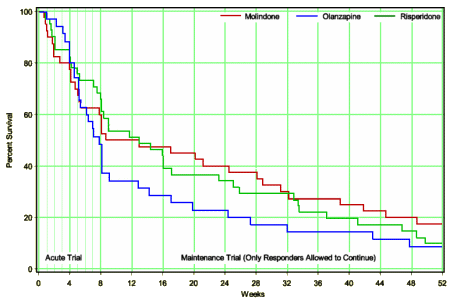
from C.A.T.I.E. [for comparison]
In the adult non-industry-initiated trials like C.A.T.I.E., Olanzapine won the efficacy side but faltered badly on weight gain and other metabolic parameters. With the kids, it wasn’t superior in efficacy [probably the opposite]; it looks like some responders stopped taking it after two months; and it is a metabolic nightmare. I didn’t even look at this study to learn more about Zyprexa, but there it is in the data. I was looking at the study to see if there was anything in what the Janssen Sales Reps said that might actually be true about Risperdal. I was surprised by the fact that the Risperdal cohort gained about the same amount of weight as the Zyprexa group [I think that I’d fallen for Janssen’s hype myself – that it was more weight neutral].
Sorry, the comment form is closed at this time.