At the risk of being labeled obsessed myself, I’m still on the Gibbons et al article [Suicidal Thoughts and Behavior With Antidepressant Treatment] published on-line this month in the Archives of General Psychiatry about treatment emergent suicidality with the SSRIs. They said of their data sources for this meta-analysis, "we obtained complete longitudinal data for RCTs of fluoxetine hydrochloride conducted by Eli Lilly and Co, the Treatment for Adolescents With Depression Study of fluoxetine in children by the National Institute of Mental Health, and adult studies for venlafaxine hydrochloride conducted by Wyeth." In the abstract, they say, "Data Sources: All intent-to-treat person-level longitudinal data of major depressive disorder from 12 adult, 4 geriatric, and 4 youth randomized controlled trials of fluoxetine hydrochloride and 21 adult trials of venlafaxine hydrochloride." I wasn’t interested in the adult data, but went looking for the mentioned studies for children and adolescents other than TADS. There are four studies listed and reviewed in the FDA Medical Review for Prozac’s approval for MDD and OCD for children and adolescents in January 2003 and also in the FDA Hammonds Review in August 2004 prior to the black box warning [published full text on-line]:
| STUDY | DX | SPONSOR | YEAR | N | PBO | FLX | DURATION |
|
|
|||||||
| HCCJ | MDD | Lilly | 1984 | 40 | 19 | 21 | 6 weeks |
| X065 | MDD | NIMH? | 1991 | 96 | 48 | 48 | 8 weeks |
| HCJE | MDD | Lilly | 1998 | 219 | 110 | 109 | 13 weeks |
| HCJW | OCD | Lilly | 1999 | 103 | 71 | 32 | 9 weeks |
| subtotal | 458 | 248 | 210 | ||||
| TADS | MDD | NIMH | 2000 | 433 | 206 | 227 | 36 weeks |
| total | 891 | 454 | 437 | ||||
| … | |||||||
| subtotal | 458 | 248 | 210 | ||||
| TADS | MDD | NIMH | 2000 | 439 | 216 | 223 | 12 weeks |
| total | 897 | 464 | 433 | ||||
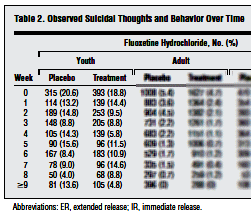
No Link Between Antidepressant and Suicide in Kids
Study Calls FDA Black Box Warning Into Question
Medscape
by Fran Lowry
February 21, 2012
A close look at all randomized, placebo-controlled trials of the antidepressant fluoxetine has failed to find evidence that the drug increases the risk for suicide in youths. And a similar look at all trials of fluoxetine and venlafaxine in adults has concluded that both antidepressants decrease depressive symptoms as well as suicidal thoughts and behavior in adult and geriatric patients. The findings, reported online February 6 in the Archives of General Psychiatry, call into question the wisdom of the US Food and Drug Administration’s 2004 decision to issue a "black box warning" about the purported link between antidepressant use and suicide, lead author Robert D. Gibbons, PhD, from the University of Chicago in Illinois, told Medscape Medical News. Dr. Gibbons, who was on the FDA’s advisory committee that voted in favor of the black box warning, said he was very concerned about the validity of the data that prompted the affirmative vote…More Harm Than Good
"I think this is a very important analysis that Robert Gibbons has published," Charles F. Reynolds III, MD, professor of geriatric psychiatry from the University of Pittsburgh, told Medscape Medical News. "Robert is one of the world’s most distinguished biostatisticians in mental health research and has had a long-standing interest in the questions of suicide, particularly the risk for and protective factors against suicide." Dr. Gibbons and his group have shown that the use of antidepressant medication is protective against suicidal ideation in adults and senior adults and that the effects on suicidal ideation are mediated through the antidepressant effects on general depressive symptomatology, Dr. Reynolds pointed out."This makes sense if you understand that depression is a major risk factor for suicidal ideation and behavior, and so, by relieving the mental illness, in this case the depression, you remove one of the main drivers of suicidal ideation. This is very important to understand because there’s been considerable concern about whether antidepressant medication might activate suicidal ideation in some people, particularly in young adults. But what he is finding is that the effects of the medication are neutral with respect to suicidal ideation in youth and protective in middle age and older adults." Dr. Reynolds said he often worries that the black box warning has done more harm than good. "The black box warning might have prevented the medically appropriate use of antidepressants in teenagers. I am very happy to see this study come out," he said.
Sorry, the comment form is closed at this time.