Antipsychotics for Children and Young Adults:
A Comparative Effectiveness Review
by Seida JC, Schouten JR, Boylan K, Newton AS, Mousavi SS, Beaith A, Vandermeer B, Dryden DM, and Carrey N.
Pediatrics 2012 Feb 20. [Epub ahead of print]
BACKGROUND AND OBJECTIVE: Despite increasing on-label and off-label use of antipsychotics, prescribing antipsychotics to children remains controversial due to uncertainty of their relative benefits and safety. We systematically reviewed the effectiveness and safety of first- [FGA] and second-generation antipsychotics [SGA] for patients aged ≤24 years with psychiatric and behavioral conditions.
METHODS: We searched 10 databases from January 1987 to February 2011, gray literature, trial registries, and reference lists. Two reviewers independently selected studies, assessed methodologic quality, and graded the evidence. One reviewer extracted, and a second verified, data. We summarized findings qualitatively and conducted meta-analyses when appropriate.
RESULTS: Sixty-four trials and 17 cohort studies were included. Most trials had a high risk of bias; cohort studies had moderate quality. All comparisons of FGAs versus SGAs, FGAs versus FGAs, and FGAs versus placebo had low or insufficient strength of evidence. There was moderate strength of evidence for the following comparisons. Olanzapine caused more dyslipidemia and weight gain, but fewer prolactin-related events, than risperidone. Olanzapine caused more weight gain than quetiapine. Compared with placebo, SGAs improved clinical global impressions [schizophrenia, bipolar and disruptive behavior disorders] and diminished positive and negative symptoms [schizophrenia], behavior symptoms [disruptive behavior disorders], and tics [Tourette syndrome].
CONCLUSIONS: This is the first comprehensive review comparing the effectiveness and safety across the range of antipsychotics for children and young adults. The evidence on the comparative benefits and harms of antipsychotics is limited. Some SGAs have a better side effect profile than other SGAs. Additional studies using head-to-head comparisons are needed.
While the article above is about that risk/benefit data, a meta-analysis with the subtext question of bias in the literature, this post is really about my own acquired bias over the last several years. It’s in the journal, Pediatrics, not the psychiatric literature, and it was compiled by a group of Canadians from a variety of disciplines. I realized when I ran across it that I was looking it up expecting to trust what I read before I even read it because it came from outside the danger zone – the pharmaceutical industry, the psychiatric literature, my own country, and away from our halls of academia. I think it’s a sad commentary that I would have that reaction – looking outside my former reliable haunts for the trustable information.
Two reviewers independently assessed the methodologic quality of studies and resolved discrepancies through consensus. We evaluated trials using the Cochrane Collaboration Risk of Bias tool and cohort studies using a modified Newcastle-Ottawa Scale. The source of funding was recorded for all studies.
Two reviewers graded the strength of the body of evidence using the AHRQ GRADE approach. The evidence was graded separately for each comparison and outcome. Four domains were assessed: risk of bias, consistency, directness, and precision. Based on the individual domains, we assigned an overall strength of evidence [SOE] grade of high, moderate, low, or insufficient. When no studies were available for an outcome or the evidence did not permit estimation of an effect, we rated the SOE as insufficient.
To determine the overall SOE score, we first considered the risk of bias domain. RCTs with a low risk of biaswere initially considered to have a “high” SOE, whereas RCTs with high risk of bias and well conducted cohort studies received an initial grade of “moderate” SOE. Low quality cohort studies received an initial grade of “low” SOE. The SOE was then upgraded or downgraded depending on the assessments of the remaining domains.
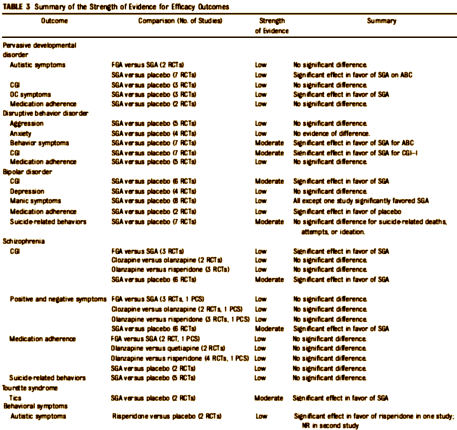
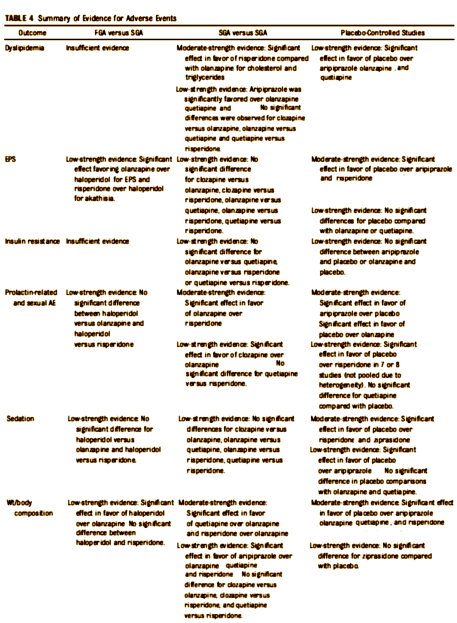
| Let me hasten to add that I’ve removed all the numeric data from both tables for fit only. That’s not to say that those numbers are unimportant. Anyone wanting to use this study for any scientific purpose should look at the tables in full. My intent is not to degrade this article, quite the opposite. I want to call attention to it because I think they did a fair and honest job of vetting a literature so suffused with opinion and bias that it has been almost impossible to interpret. |
There’s a whole lot of low Strength of Evidence in both efficacy and adverse effects. We’ve had almost two decades of controversy around this topic – studies, articles in medical journals, blogs, articles in newspapers, FDA/Industry debates, suits in our courts, and this is the bottom line, "The evidence on the comparative benefits and harms of antipsychotics is limited."
I said this blog post is about my own bias. Up front, I’m not thrilled about giving big medicines to little people, but I know that clinical situations arise where that becomes a real question. My point is that a doctor in those situations enters with very little in the way of solid risk/benefit information to help make that kind of sticky decision, to give to parents to help them know what to do, or especially in situations like kids in foster care or institutions. That’s not the way things are supposed to work – not with potentially toxic drugs that have been around as long as these.
I find it really interesting that the American Academy of Pediatrics focuses on behavioral techniques for helping kids with conduct disorders. They say if those don’t work, stimulants of the type used for ADHD may be prescribed, and if those are ineffective “other drugs may be used.
The NIH, on the other hand, notes that the drugs most commonly used are atypical antipsychotics, mood stabilizers, and alpha agonists like clonidine.
How did the atypical antipsychotics come to be so widely used?
http://www.healthychildren.org/English/health-issues/conditions/emotional-problems/pages/Disruptive-Behavior-Disorders.aspx?nfstatus=401&nftoken=00000000-0000-0000-0000-000000000000&nfstatusdescription=ERROR%3a+No+local+token
Sorry, here’s the link to the NIH info
http://store.samhsa.gov/shin/content//SMA11-4634CD-DVD/MedicationManagementChild-IDBD.pdf