
Duloxetine was created by Lilly researchers. David Robertson, David Wong, a co-discoverer of fluoxetine, and Joseph Krushinski are listed as inventors on the patent application filed in 1986 and granted in 1990… Initial trials conducted in patients using regimens of 20 mg/day or less did not convincingly demonstrate its efficacy and the dose was increased to as high as 120 mg in subsequent clinical trials.
In 2001 Lilly filed a New Drug Application [NDA] for duloxetine with the US Food and Drug Administration [FDA]. However, in 2003 the FDA "recommended this application as not approvable from the manufacturing and control standpoint" because of "significant cGMP [current Good Manufacturing Practice] violations at the finished product manufacturing facility" of Eli Lilly in Indianapolis. Additionally, "potential liver toxicity" and QTc interval prolongation appeared as a concern. The FDA experts concluded that "Duloxetine can cause hepatotoxicity in the form of transaminase elevations. It may also be a factor in causing more severe liver injury, but there are no cases in the NDA database that clearly demonstrate this. Use of duloxetine in the presence of ethanol may potentiate the deleterious effect of ethanol on the liver." The FDA also recommended "routine blood pressure monitoring" at the new highest recommended dose of 120 mg, "where 24% patients had one or more blood pressure readings of 140/90 vs. 9% of placebo patients."
After the manufacturing issues were resolved, the liver toxicity warning included in the prescribing information, and the follow-up studies showed that duloxetine does not cause QTc interval prolongation, duloxetine was approved by the FDA for depression and diabetic neuropathy in 2004. In 2007 Health Canada approved duloxetine for the treatment of depression and diabetic peripheral neuropathic pain… The FDA approved duloxetine for the treatment of generalized anxiety disorder in February 2007.
| STUDY | COMPARATOR | LOCATION | OUTCOME |
| HMATa | PAXIL | US | POSITIVE |
| HMATb | PAXIL | US | FAILED |
| HMBHa | – | US | POSITIVE |
| HMBHb | – | US | POSITIVE |
| HMAQa | PROZAC | NON-US | FAILED |
| HMAQb | PROZAC | NON-US | FAILED |
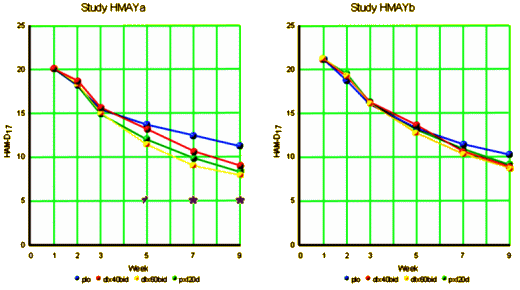
| HMAYa | PAXIL | NON-US | POSITIVE |
| HMAYb | PAXIL | NON-US | FAILED |
These two graphs are the kind of separations seen in the rest of these studies.
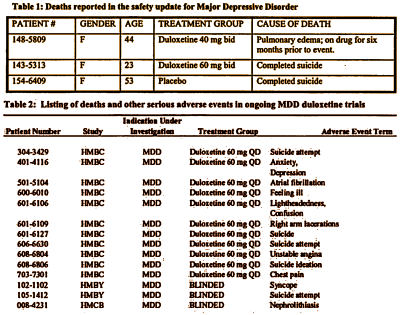
And, of course, no one likes reading this:

Desvenlafaxine … is an antidepressant of the serotonin-norepinephrine reuptake inhibitor class developed and marketed by Wyeth [now part of Pfizer]. Desvenlafaxine is a synthetic form of the major active metabolite of venlafaxine [sold under the brand names Effexor and Efexor]. It is being targeted as the first non-hormonal based treatment for menopause.
UNITED STATES: Wyeth announced on 23 January 2007 that it received an "approvable" letter from the Food and Drug Administration for desvenlafaxine. Final approval to sell the drug was contingent on a number of things, including:
• a satisfactory FDA inspection of Wyeth’s Guayama, Puerto Rico facility [manufacturer];
• several post-marketing commitments;
• clarity by Wyeth around the company’s product education plan for physicians and patients;
• approval of desvenlafaxine’s proprietary name, Pristiq.
The FDA approved the drug for antidepressant use in February 2008, and was to be available in US pharmacies in May 2008.
CANADA: On February 4, 2009, Health Canada approved use of desvenlafaxine for treatment of depression in Canada. Pristiq is now available in Canadian pharmacies.
EUROPEAN UNION: In the European Union desvenlafaxine succinate has not been approved for any indication. In 2008, Wyeth withdrew its application for Ellefore, the product under review for treatment of major depressive disorder. The reasons given by Wyeth, and comments regarding the findings of the reviewing agency are provided in a "question and answer" format document The European Medicines Agency explained that the [Committee for Medicinal Products for Human Use] had some concerns and was of the provisional opinion that Ellefore could not have been approved for the treatment of major depressive disorder [and] overall, the effectiveness of Ellefore had not been shown convincingly. In relation to its parent substance, venlafaxine, desvenlafaxine seemed to be less effective with no advantages in terms of safety and tolerability.
The FDA Review for approval was based on two identical studies:
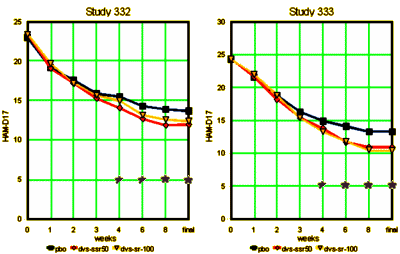
Something else we don’t much like reading:

As many of these graphs of Clinical Trials as I’ve compiled and posted, I’m still not used to them. Even in my own experience of prescribing these drugs, the graphs don’t make any sense. The small differences confound me. I’ve never prescribed these two, but I’ve written enough prescriptions for SSRIs [almost always Citalopram if it’s up to me], and patients either say, "I can’t tell any difference" or "yeah, it’s helping" which just doesn’t fit a 2 or 3 point rise on a HAM-D17. I assume these curves have a lot to do with the Clinical Research Organizations way of recruiting subjects, but who really knows? I sure don’t. But every time I paste the tables into a spread sheet and do the graph, I’m jarred by what I see.
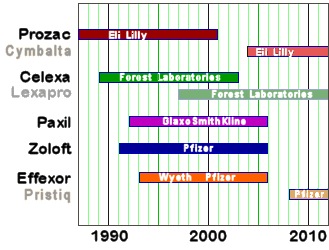
It looks like Lilly and Pfizer were aiming for when their existing drug’s patents expired, but the FDA messed up the schedule [not shown is Forest‘s recent acquisition, the newly approved Viibryd – another SSRI]:
Mickey, see the article http://www.slate.com/articles/health_and_science/medical_examiner/2005/09/drug_secrets.html about Traci Johnson, college student who killed herself in the Lilly lab while healthy, and only earning money for college by trialing Cymbalta, which began as a drug for incontinence…