Sally Laden certainly had her work cut out for her with the study below. It looks like what GSK had in mind was to show that in bipolar patients on Lithium for Mania who got depressed, Paxil was the drug to use [rather than an old Tricyclic]. So they did a clinical trial at all the right centers to find out. But it didn’t go as planned.
U. of Pennsylvania Absolves Psychiatry Chairman of Ghostwriting Complaint
Chronicles of Higher Education
by Paul Basken
February 29, 2012
The University of Pennsylvania has concluded that the chairman of its psychiatry department and a colleague let their names be listed among the authors reviewing a medicine in a journal article that was actually written by the drug’s manufacturer. The university, however, has decided to take no punitive action against the chairman, Dwight L. Evans, and an associate professor of psychiatry, Laszlo Gyulai, for the 2001 article in The American Journal of Psychiatry. Penn noted…
The University of Pennsylvania has denied allegations made by one of its professors that several other academics – including his department chair – allowed their names to be added to a medical journal manuscript, but gave control of the contents to GlaxoSmithKline, according to his attorney. The study, which was funded by the drugmaker and the National Institutes of Health, looked at the impact of the Paxil antidepressant on patients with bipolar disorder.At the same time, the university has acknowledged a claim by the professor, Jay Amsterdam, that the 2001 study was ghostwritten by Scientific Therapeutics Information, his attorney tells us. However, he says the university is not planning on taking any action in connection with the ghostwriting. The study, which was published by the American Journal of Psychiatry [see here], did not mention that STI played any role [here is an email in which STI employee Sally Laden discusses that she would work on the paper].
“They said his allegations were not meritorius, although they did find that the publication at issue was ghostwritten,” says Bijan Esfandiari, the attorney, citing a letter and other documents he received from the university. “They acknowledged that a marketing firm was involved in drafting, and everything associated with, the issue. But in response to our complaint, they said that, at the time these events took place, which was between 1998 and 2001, ghostwriting was standard practice and everyone was doing this, so therefore, we’re not going to punish any individuals”…
Amsterdam, 62, last year filed a complaint with the federal Office of Research Integrity charging scientific misconduct. In a letter to the ORI, he alleged “the published manuscript was biased in its conclusions, made unsubstantiated efficacy claims and downplayed the adverse event profile of Paxil.” He also claimed he was a co-principal investigator, but was excluded from the final data review, analysis and publication [here is the letter]…
Double-blind, placebo-controlled comparison of imipramine and paroxetine in the treatment of bipolar depression.
by Nemeroff CB, Evans DL, Gyulai L, Sachs GS, Bowden CL, Gergel IP, Oakes R, Pitts CD.
American Journal of Psychiatry. 2001 158[6]:906-912.
SOURCE: Department of Psychiatry and Behavioral Sciences, Emory University School of Medicine…
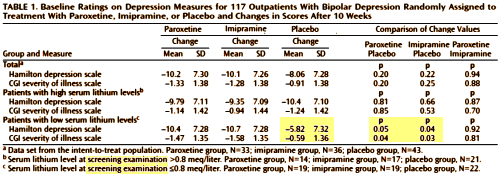
OBJECTIVE: This study compared the efficacy and safety of paroxetine and imipramine with that of placebo in the treatment of bipolar depression in adult outpatients stabilized on a regimen of lithium.
METHOD: In a double-blind, placebo-controlled study, 117 outpatients with DSM-III-R bipolar disorder, depressive phase, were randomly assigned to treatment with paroxetine [N=35], imipramine [N=39], or placebo [N=43] for 10 weeks. In addition to lithium monotherapy, patients may have received either carbamazepine or valproate in combination with lithium for control of manic symptoms. Patients were stratified on the basis of trough serum lithium levels determined at the screening visit (high: >0.8 meq/liter; low: < 0.8 meq/liter). Primary efficacy was assessed by change from baseline in scores on the Hamilton Rating Scale for Depression and the Clinical Global Impression illness severity scale.
RESULTS: Differences in overall efficacy among the three groups were not statistically significant. For patients with high serum lithium levels, antidepressant response at endpoint also did not significantly differ from placebo. However, both paroxetine and imipramine were superior to placebo for patients with low serum lithium levels. Compared to imipramine, paroxetine resulted in a lower incidence of adverse events, most notably emergence of manic symptoms.
CONCLUSIONS: Antidepressants may not be useful adjunctive therapy for bipolar depressed patients with high serum lithium levels. However, antidepressant therapy may be beneficial for patients who cannot tolerate high serum lithium levels or who have symptoms that are refractory to the antidepressant effects of lithium.
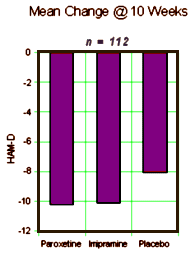 Note that they mention the DSM-IIIR [the DSM-IV came out in 1994] so this must be really some old data. Whenever it was done, they took Manic patients on Lithium [among other things] who were Depressed, and gave them antidepressants [either Imipramine, Paroxetine, or Placebo] and followed them for ten weeks doing HAM-Ds, CGIs, and drawing Lithium levels at weeks 1-6, 8, and 10. It was a bust [which is why it probably sat on a shelf for a while]. Neither drug beat placebo [statistically], and the drugs were about equal. No way to spin that one. Then someone had a bright thought. "Wait, I’ve got an idea, let’s look at the Lithium Levels! If we split them up based on the initial Lithium levels, that might be a way to see some significance." And so that’s what they did. What they got by doing that was no great testimonial for their drug, but it was at least significant – both Imipramine and Paxil beat Plabebo if, and only if, the Lithium Level was under 0.8.:
Note that they mention the DSM-IIIR [the DSM-IV came out in 1994] so this must be really some old data. Whenever it was done, they took Manic patients on Lithium [among other things] who were Depressed, and gave them antidepressants [either Imipramine, Paroxetine, or Placebo] and followed them for ten weeks doing HAM-Ds, CGIs, and drawing Lithium levels at weeks 1-6, 8, and 10. It was a bust [which is why it probably sat on a shelf for a while]. Neither drug beat placebo [statistically], and the drugs were about equal. No way to spin that one. Then someone had a bright thought. "Wait, I’ve got an idea, let’s look at the Lithium Levels! If we split them up based on the initial Lithium levels, that might be a way to see some significance." And so that’s what they did. What they got by doing that was no great testimonial for their drug, but it was at least significant – both Imipramine and Paxil beat Plabebo if, and only if, the Lithium Level was under 0.8.: 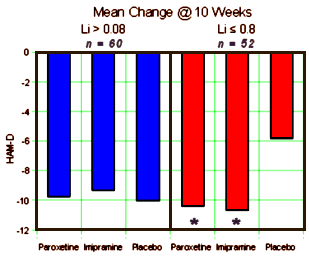
[truncated for clarity]
First, the study failed to recruit a sufficient patient sample size to adequately test the primary efficacy outcome measure. The primary efficacy outcome measure failed to show superiority of either antidepressant drug treatment compared to placebo. This important information was not reported in the manuscript. The authors then relied on post hoc analyses of subsets of the data to find a favorable result for the antidepressant Paxil. Specifically, this result was accomplished by sub-dividing patient cohorts for each treatment into sub-groups of "high" (Le., ~8.0 mEqjL) versus "low" (Le., <8.0 mEqjL) baseline serum lithium levels after the primary data analyses were found to be. negative. This post hoc data presentation was then presented as the primary study finding, and gave the false impression that one group of patients with low lithium levels (who may be unable to tolerate higher lithium levels) showed superior benefit with Paxil versus placebo (compared to imipramine versus placebo).
Moreover, patients with "low" lithium levels were presented as being a distinct patient group who were somehow different from patients in the "high" lithium level group. In fact, this was a disingenuous distinction because all of the patients in the study had what were considered to be adequate and clinically therapeutic lithium levels, or they would have been discontinued from the trial. Moreover, this sub-division of treatment cohorts into "high" versus "low" lithium level groups was not clinically meaningful and these data were added to the manuscript to produce a favorable outcome finding for promoting Paxil (in a study that was otherwise negative in its findings and that recruited an insufficient patient sample size to accurately test the null hypothesis for the primary efficacy measures). Second, the published manuscript downplayed a well-known (and potentially dangerous) adverse event profile of Paxil. For example, the manuscript did not report any mania ratings (e.g., Young Mania Rating Scale), although the results section did hote that end-point mania analyses were performed. The manuscript portrayed Paxil as being safe and producing no manic symptoms or manic episodes (in either the entire Paxil-treated patient group or in the "high" or "low" lithium level sub-groups), a finding which was not supported by available clinical or research evidence in 2001 (or subsequent to that date). As a result, the stated findings suggest that Paxil is a safe and well tolerated alternative to imipramine (the other antidepressant used in the study) which appeared to cause manic symptoms in both the "high" and "low" lithium level patient subgroups. Thus, these purported findings ran completely counter to almost all available clinical and research findings up to 2001 (and subsequent to that date), and suggested a treatment approach for bipolar depression (i.e., Paxil) which contradicted much of the available clinical and research evidence, as well as most published practice guidelines for treating bipolar type I depression.
Third, the results in the published manuscript emphasized a substantial side effect profile for imipramine while minimizing and down-playing the side effect profile of Paxil. For example, the manuscript emphasized a substantial rate of sexual side effects for imipramine (an antidepressant drug not particularly known to produce this side effect), while down-playing the sexual side effect profile of Paxil, and suggested that there were no sexual side effects encountered with Paxil in the study. This was a grossly misleading fact which was further emphasized by the authors citing the medical literature indicting only imipramine side effects while simultaneously omitting citations from the medical literature that accurately report the incidence of Paxil sexual side effects. In this regard, the published manuscript stated that "patients treated with imipramine reported a higher incidence of abnormal ejaculation (18.8%) and impotence (25.0%) than did patients receiving paroxetine (0.0% and 6.3%, respectively) or placebo (5.0% and 0.0%, respectively)". Moreover, in the discussion section of the published manuscript, this "finding" is further supported by literature citing the high sexual side effect rate with imipramine while providing no citations for Paxil-induced side effects – even though Paxil’s sexual side effects were well known at the time of publication. In fact, the side effect bias favoring Paxil was so supportive and contrary to the available medical literature in 2001 that it would be reasonable for a reader to wonder whether SmithKline Beecham, Inc. actually provided the side effect citations to the" authors" for publication in the published manuscript.
I have come to NOT expect much from Pennsylvania…….
Written from Florida………