In June 2009, the Institute of Medicine held a Workshop in Washington DC, CNS CLINICAL TRIAL: SUICIDALITY AND DATA COLLECTION [available on-line]. It was sponsored by an array of foundations and pharmaceutical companies, co-chaired by Dr. William Potter of Merck Research Laboratories and Dr. Robert Gibbons of the University of Illinois at Chicago. The topic was the FDA’s "black box" warning added to antidepressants in late 2004 [expanded in 2007]. All the principals were on the program – including Dr. Robert Gibbons and his senior author on these recent papers, Dr. J. John Mann, Dr. Kelly Posner of the C-SSRS, Dr. Thomas Laughren of the FDA – it was a regular Who’s Who from the world of suicidology. Obviously, the FDA meta-analysis was on the front burner [FDA Hammonds Review, published full text on-line] and Kaizar’s extension [Do antidepressants cause suicidality in children? A Bayesian meta-analysis.]. This is from the latter listing all the clinical trials in youths highlighted by yours truly [available on-line]:
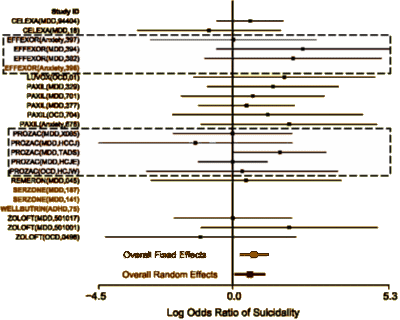
If you look at the Prozac trials, they might look familiar from my futile attempts to figure out Dr. Gibbon’s data in advance [coming soon?…]. Three out of four of his selected studies are on the list [X065, TADS, and HCJE]. As you can see, two of those are suicidality neutral [X065 and HCJE] but TADS is definitely not. Nowhere to be seen is Study LYAQ which he used in his recent meta-analysis.
And as long as we’re looking at this slide, Dr. Gibbons’ recent articles used data from the Effexor Adult studies kindly provided by Wyeth [Pfizer]. One wonders why he didn’t also include the data from the Effexor studies of MDD in youngsters? There are two studies [382 and 394] right up there, surely available for his meta-analysis. Well, I can think of two reasons right off the cuff. First, they are anything but suicidality neutral [above]. And the second is in this FDA Effexor Medical Review from 2003 – both studies were efficacy negative:
B. Efficacy Summary of Studies of MDD
Two, 8-week, multi-center parallel group randomized, double blind, placebo controlled flexible dose studies did not provide any evidence of venlafaxine’s efficacy in the treatment of MDD in children. These studies employed doses ranging from 37.5 to 225-mg/day. They were adequately powered studies with 161 (103 completing) patients in study 382 and 193 patients (143 completing) in study 394. There were no differences between placebo and drug treatment groups at week eight (8) via the last-observation-carried-forward (LOCF) on-therapy evaluation (382: P=0.338; 394: P=0.386).
Two, 8-week, multi-center parallel group randomized, double blind, placebo controlled flexible dose studies did not provide any evidence of venlafaxine’s efficacy in the treatment of MDD in children. These studies employed doses ranging from 37.5 to 225-mg/day. They were adequately powered studies with 161 (103 completing) patients in study 382 and 193 patients (143 completing) in study 394. There were no differences between placebo and drug treatment groups at week eight (8) via the last-observation-carried-forward (LOCF) on-therapy evaluation (382: P=0.338; 394: P=0.386).
I went back to Dr. Gibbons et al’s recent articles to review their descriptions of the datasets. There’s no reason given for not including the Effexor studies for kids, at least none I could locate. Both Studies [382 and 394] used the CDRS-R rating scale [here] and had adequate number of subjects [here]:.
To help determine what impact antidepressants have on the course of depression and suicidal thoughts and behavior in different age groups, we obtained complete longitudinal data for RCTs of fluoxetine hydrochloride conducted by Eli Lilly and Co, the Treatment for Adolescents With Depression Study of fluoxetine in children by the National Institute of Mental Health, and adult studies for venlafaxine hydrochloride conducted by Wyeth.
All trials were double-blind, placebo-controlled RCTs. For fluoxetine, we reanalyzed studies that included 30 or more patients and used the HAM-D for adults and geriatric patients or the Children’s Depression Rating Scale–Revised (CDRS-R) for youths. The only trial exclusions were 1 adult study that did not use the HAM-D, 1 study judged to be invalid, and 1 youth study that did not use the CDRS-R. Fluoxetine trial data from the Treatment for Adolescents With Depression Study11 were obtained from the National Institute of Mental Health; individual level data for the remaining 12 adult studies, 4 geriatric studies, and 3 youth studies were obtained from Eli Lilly and Co. We obtained patient-level data from Wyeth for all available adult venlafaxine RCTs (11 with venlafaxine immediate release [IR] and 10 with venlafaxine extended release [ER]).
So I can think of no conclusion other than the fact that they didn’t like how the Effexor Trials came out [no efficacy, high suicidality]. In my book, leaving out the Effexor child and adolescent studies and unearthing the Prozac LYAQ Strattera Study to include [come rest a while I…, come rest a while II…, come rest a while III – the finale…] can’t be seen as anything other than "cherry-picking" data to get it to come out like they wanted it to come out. If anybody can think of any other possible reasons, please let me know.
There’s more to say about the workshop, but frankly my eyes are crossing from FDA document fatigue, so I’ll go rest a while…
A must-read from Scientific American –
http://blogs.scientificamerican.com/cross-check/2012/03/05/are-psychiatric-medications-making-us-sicker/
Duane
As part of the European evaluation of Prozac to children the Dutch Medicines Evaluation Board (CBG), being Rapporteur, in October 2005 issued “Prozac (fluoxetine) – Paediatric Indication, Rapporteurs’ Assessment Report” It is an incredible report. It begins with the words: “It is not recommended to grant an indication to fluoxetine for the treatment of depression in children and adolescents because the benefit/risk balance in the claimed indication is deemed negative.†Note that this evaluation is made nearly three years after FDA (January 2003) approved Prozac for children. Please note that the evaluation is made after the Dutch Medicines Evaluation Board asked for and received answers to a number of follow-up questions from Eli Lilly. The approval of Prozac for children was later pushed through by the UK MHRA and the Swedish Medical Products Agency, with arguments that it would give good chances for “better post marketing surveillance in these populations and possibilities to request further studiesâ€, which of course never happened.
But read what the Dutch CBG had to say about Lilly’s application and submitted studies: http://jannel.se/Prozac-HollandAR.pdf