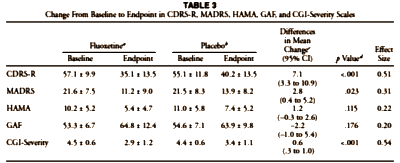
The results do not support previous findings that antidepressants show little benefit except for severe depression. The antidepressants fluoxetine and venlafaxine are efficacious for major depressive disorder in all age groups, although more so in youths and adults compared with geriatric patients. Baseline severity was not significantly related to degree of treatment advantage over placebo.
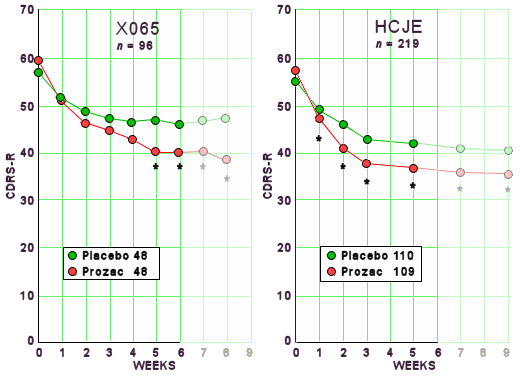
X065
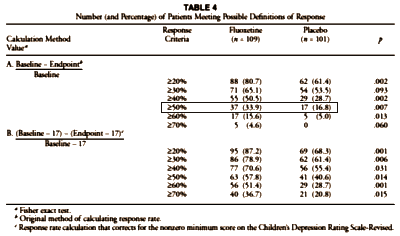
Using the intent to treat sample, 27 [56%] of those receiving fluoxetine and 16 [33%] receiving placebo were rated "much" or "very much" improved on the Clinical Global Impressions scale at study exit [Chi-squared=5.1, df=1, P=.02]. Significant differences were also noted in weekly ratings of the Children’s Depression Rating Scale-Revised after 5 weeks of treatment [using last observation carried forward]. Equivalent response rates were found for patients aged 12 years and younger [n=48] and those aged 13 years and older [n=48]. However, complete symptom remission [Children’s Depression Rating Scale-Revised] occurred in only 31% of the fluoxetine-treated patients and 23% of the placebo patients.
HCJE
Significantly more fluoxetine treated patients [41.3%] than placebo-treated patients [19.8%] met the prospectively defined criteria for remission [p < .01].
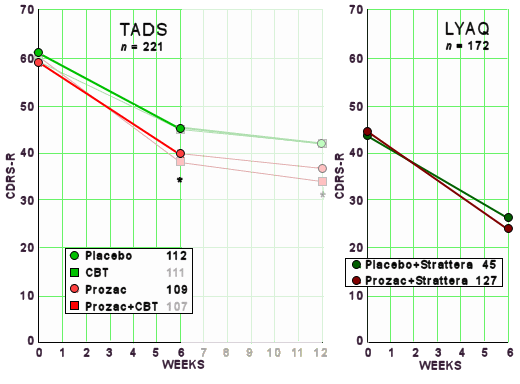
TADS
The rate of remitted subjects at the end of treatment, based on the dichotomized CDRS-R, was 102 of 439, or 23%. There was a significant overall treatment [Wald x2= 17.08, df = 3, p = .0007] and site [Wald x2 = 21.5, df =12, p = .04] effect. More specifically, the COMB group with a 37% remission rate was superior to all of the other treatment conditions [FLX: 23%, p = .02; CBT: 16%, p = .0004; PBO: 17%, p = .0009]. However, the FLX, CBT, and PBO rates were not statistically different from one another [p 9 .05]….The overall rate of response, defined as a CGI-I score of 1 or 2 in the intention-to-treat sample was 229 of 439, or 52% [TADS, 2004]. As previously reported, response rates by treatment arm were as follows: 71.0% [COMB], 60.6% [FLX], 43.2% [CBT], and 34.8% [PBO].
LYAQ
GIBBONS et al
| Youth Studies of Fluoxetine In youth studies of fluoxetine, the estimated average rates of change over 6 weeks were −15.96 CDRS-R units for placebo and −20.58 CDRS-R units for fluoxetine [MMLE=−4.62; SE=1.26; P<.001], indicating 29.0% greater improvement for fluoxetine. The estimated response rates were 29.8% for fluoxetine vs 5.7% for placebo [OR=6.66; 95% CI, 3.07- 14.48; P<.001; NNT=4.16]. Remission rates were 46.6% vs 16.5% for fluoxetine and placebo, respectively [OR=4.23; 95% CI, 2.64-6.77; P<.001;NNT=3.33]. The finding of higher remission rates than response rates questions the validity of the CDRS-R remission threshold score of 28. No effect of baseline severity on treatment efficacy was found [P=.90]. For patients with low severity, the rates of change in symptoms were −17.60 CDRS-R units for fluoxetine vs −12.56 CDRS-R units for placebo. For patients with high severity, the rates of change were −28.86 CDRS-R units for fluoxetine vs −24.40 CDRS-R units for placebo. The estimated differences were 5.04 CDRS-R units [95% CI, 2.56 to 7.52] for low severity and 4.45 CDRS-R units [95% CI, −0.58 to 9.49] for high severity. Estimated response rates for treated vs placebo receiving patients were 23.0% vs 3.2% [difference of 19.8%], respectively, for low severity and 40.2% vs 17.2% [difference of 23.0%], respectively, for high severity. Estimated remission rates for treated vs placebo-receiving patients were 54.1% vs 19.4% [difference of 34.7%], respectively, for low severity and 28.9% vs 7.5% [difference of 21.4%], respectively, for high severity. |
The outcome criteria for Gibbons et al are:
Using the values from above, we can construct the following table comparing the reported results between the pertinent input studies [X065, HCEJ, and TADS] and Gibbons’ outcome for the meta-analysis of all four studies. Recall that the lower the Number Needed to Treat, the more efficacious the treatment [NNT = 1 ÷ diff]. And it all comes down to the red numbers in the table below – the outcome variables [NNT] from the original studies that fit the same criteria used by Gibbons:
| X065 | HCEJ | TADS | GIBBONS | |||||||||||||
| PARAMETER | Pbo | Flx | diff | Pbo | Flx | diff | Pbo | Flx | diff | Pbo | Flx | diff | ||||
|
|
||||||||||||||||
| Response CGI | 33% | 56% | 23% | – | – | – | 34.8% | 60.6% | 25.8% | – | – | – | ||||
| NNT | 4.35 | – | 3.88 | – | ||||||||||||
| Response CDRS-R | – | – | – | 16.8% | 33.9% | 17.1% | – | – | – | 5.7% | 29.8% | 24.1% | ||||
| NNT | – | 5.85 | – | 4.15 | ||||||||||||
| Remission Rate |
22.9% | 31.3% | 8.4% | 19.8% | 41.3% | 21.5% | 17% | 23% | 6% | 16.5% | 46.6% | 30.1% | ||||
| NNT | 11.9 | 4.65 | 16.67 | 3.33 | ||||||||||||
As I said, there was no way for me to adjust the number to make up for the fact that Gibbons didn’t use all the data, but I still think if anything it would decrease efficacy. For the numbers I could compute, Gibbon’s outcome was better that any of the studies he used for his meta-analysis. And that’s before the decrease from the negative study LYAQ and whatever efficacy he lost by cutting off the end of the others. I find that remarkable impossible. How can the meta-analysis be better than any of the pieces used to derive it? better for remission in all cases? better in the one case where we can make direct response rate comparisons? better absent the effect of a large negative study with 24% of the overall subject pool?
Gibbons study is all about sending out a marketing message & grabbing press headlines….the numbers don’t need to add up…it’s the same old statistical slight of hand that has been used now for decades getting these drugs approved and into the mega sales market place…you could prove that this study or analysis was completely bogus; and it might get a quick paragraph in the back of some Wednesday morning addition of the Timbuktu newspaper..sadly, just how it works…
on a brighter note…have you seen this article in the Washington Post.. http://www.washingtonpost.com/national/health-science/antipsychotic-drugs-grow-more-popular-for-patients-without-mental-illness/2012/02/02/gIQAH1yz7R_story.html
Yuk! Good for Adriane…
As for Gibbons – I take your point, but hope springs eternal, because the opposite it to just watch it happen like people did for twenty five years, and that sure didn’t help…
EVIDENCE OF ORCHESTRATED CAMPAIGN TO SELL AD’s TO CHILDREN
From: Journal of Child and Adolescent Psychopharmacology
Sent: Wednesday, March 07, 2012
Subject: More Effective Treatments Urgently Needed for Adolescent Depression
PRESS RELEASE FROM MARY ANN LIEBERT, INC., PUBLISHERS
A state-of-the-art issue reporting on the latest research findings on antidepressant medications combined with appropriate therapeutic strategies has been published by
Journal of Child and Adolescent Psychopharmacology
Special Issue on Psychopharmacology of Adolescent Depression
http://online.liebertpub.com/toc/cap/22/1
Featuring some of our favourite KOLs: Wagner, Keller, Emslie, Brent, Birmaher…
This (unusually) free issue, guest edited by G.J. Emslie, MD, lead author in Gibbons’ Lilly Fluoxetine trials, in close collaboration with Gibbons “safe†and “effective†deceipts (aided and abetted by Arch Gen Psych) appears directly intended to “neutralise†the Black Box warning, so as to persuade clinicians and parents/patients that antidepressants are safe and effective for adolescents, increasing prescription and consumption, hence rendering the warning “sales neutralâ€, ahead of Pristiq and Cymbalta’s FDA indication push. This campaign is likely also intending to persuade those in the FDA making this decision to approve the indication.
This (unusually) free issue really pushes the case to child psychiatrists. In this issue Guest Editor: G.J. Emslie, MD states “Barriers to effective treatments include public perceptions of effectiveness and safety of treatments†– and they’re working to overcome these unhelpful-to-marketing-perceptions.
Dr. Emslie receives research grant support from Biobehavioral Diagnostics Inc., Eli Lilly, Forest Laboratories, GlaxoSmithKline, and Somerset; has been a consultant for Biobehavioral Diagnostics Inc., Eli Lilly, Forest Laboratories, INC Research Inc., Lundbeck, Pfizer, Seaside Therapeutics, Shire Pharmaceuticals, and Wyeth; and has been on the Speakers Bureau for Forest Laboratories.
See video of Emslie, at the same table with Gibbons, laying out the campaign here:
http://www.medscape.org/viewarticle/587541
A Multidisciplinary Approach to Treating Major Depressive Disorder in the Adolescent Patient (Slides With Video)- Faculty and Disclosures Graham J Emslie, MD Disclosure: has received … Vanya J Hamrin, RN, MSN, … husband’s employer is Moore Medical. Robert D Gibbons, an expert witness for Pfizer, Wyeth Pharmaceuticals, and the US Dept of Justice. This appalling pharmaceutical advertisement was “supported by an independent educational grant from Forest Laboratories.†(btw should Gibbons be noting this in his COI?)
The issue is full of articles pushing the case, including: “An Open-Label Safety and Pharmacokinetics Study of Duloxetine in Pediatric Patients with Major Depression” in which John S March, key NIMH advisor once again lies about COI “Dr. March has not engaged in promotional work, e.g., speakers bureau or training, for over 15 years.†cf prior 2006/7 disclosures of such work.
In the press release: “There are no radically new treatments on the horizon for the treatment of depression, and so we have to do better with the treatments we have available,†says Graham J. Emslie, MD, Guest Editor of the issue and Director of Child Psychiatry at University of Texas Southwestern Medical Center, Dallas. “Few youths with depression receive adequate treatment.â€
This whole deceptive campaign, which WILL harm our children, deserves the strongest response of informing and warning about this disinformation, on all platforms, journals and media, that responsible clinicians can possibly muster.
Reuters, Los Angeles Times etc don’t routinely scan Arch Gen Psych contents alerts, I’m sure. There ARE press releases responsible for the publicity. Who is sending them out? Can journalists share this information?
Question – Aside from letters, blogs etc on this issue, is there opportunity for public submissions to the FDA regarding this decision?