Texas judge finalizes $158 mln Risperdal settlement
Reuters
By Corrie MacLaggan
March 27, 2012A Texas judge on Tuesday finalized a $158 million agreement to settle a lawsuit that accused Johnson & Johnson of improperly marketing its Risperdal anti-psychotic drug to state residents in the Medicaid program. The action in Austin by state District Judge John Dietz paves the way for Johnson & Johnson to pay the money to the original plaintiff, his attorneys, the state of Texas and the federal government. It’s the largest fraud recovery ever in Texas for Medicaid, the federal-state health insurance program for low-income people."We are pleased that the settlement agreement has been brought to a close," Teresa Mueller, a spokeswoman for J&J’s Janssen Pharmaceuticals unit, said on Tuesday. "Janssen is committed to ethical business practices, and has policies in place to ensure its products are only promoted for their FDA-approved indications." The $158 million is the same amount that Johnson & Johnson said in January that it would pay. Since then, the parties have quibbled over the distribution of the money, according to Allen Jones, who raised the red flag about J&J’s marketing practices while working with the state of Pennsylvania. Johnson & Johnson declined comment on the discussions.
"The defendants wanted to have more of a say than they did on how the monies were allocated," Tom Melsheimer, a lawyer for Jones, told Reuters on Tuesday. Of the $158 million, about 31 percent will go to the federal government, 40 percent to the state of Texas, 17 percent to Jones and the rest [roughly 12 percent] is for attorneys’ fees, according to Tommy Jacks, a lawyer for Jones. The settlement will fully resolve all Risperdal-related claims in Texas, Mueller said. The agreement is specific to Texas and does not involve other state or federal Risperdal litigation.
The deal marked the first Risperdal settlement with any U.S. state. It settles claims brought by Texas in 2004 involving alleged Medicaid overpayments during the years 1994 to 2008. Jones said on Sunday that Johnson & Johnson was trying to reduce the amount that he received in what he characterized as an effort to discourage whistleblowers.
Under state law, Jones was entitled to receive between 15 and 25 percent of the settlement, said Tom Kelley, a spokesman for Texas Attorney General Greg Abbott. Jones said on Tuesday that the 17 percent he is getting is "far, far more" than what Johnson & Johnson wanted to pay but that "this has never been about the bucks." "I have done all that I can to expose Johnson & Johnson," Jones told Reuters on Tuesday. Kelley said that Texas officials "are completely happy" with the settlement and that Texas taxpayers are receiving a larger allocation than initially planned.
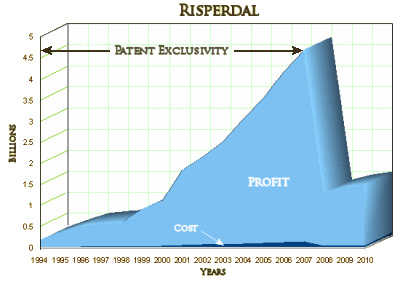
Sick people are a captive audience, no matter where they are treated. All of this money – profit, CEO golden parachute, settlement payouts is money intended to pay for the healthcare of sick people, this captive audience with no other alternatives. In this case it came largely from taxpayers paying for the healthcare of the people who can’t pay, diverted by several corrupt systems including their own State government and State supported medical schools. William Weldon is walking away with a personal mint exceeding that of any of the parties involved in this settlement. He should really be spending some quality time playing Mahjong with Bernie Madoff, wearing a blue shirt marked with his inmate number in magic marker. The magnitude of the crime is worse – sick people with no choice, taxpayers with no choice, rather than rich people actively making bad choices.
Next up, Arkansas is suing Johnson & Johnson over misrepresentations of Risperdal, with a potential award of more than $1 billion, see http://www.necn.com/03/24/12/Trial-set-for-Ark-suit-over-anti-psychot/landing.html?&apID=153ef552a5324923aba9d3d150d42edd
Risperal reproached.
Same saga here as Eli Lilly Zyprexa.
Johnson and Johnson is a trusted brand we associate with babies.
Risperdal,Zyprexa,as well as the other atypical antipsychotics, are being prescribed for children, even though this is an unapproved, off-label use. An estimated 2.5 million children are now taking atypical antipsychotics. Over half are being given them for Attention Deficit Hyperactivity Disorder,many of these foster children.
Weight gain, increases in triglyceride levels and associated risks for (life-long) diabetes and cardiovascular disease.
Lilly made $65 billion on Zyprexa!
–Daniel Haszard
*Tell the truth don’t be afraid*