|
SUBMITTED FOR PUBLICATION to the Archives of General Psychiatry
April 6, 2012
390 words, two tables, 9 references
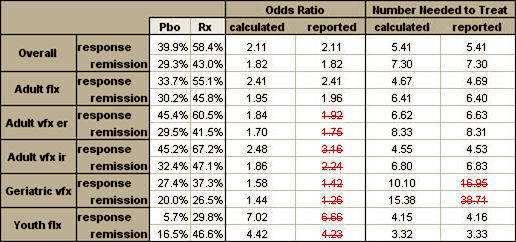
Safety and Efficacy of Antidepressant Medication in Youths Dear Sir, This meta-analysis [1][2] is deeply flawed by inappropriate data selection and computational irregularities invalidating the interpretation of their conclusions. By using only adult venlafaxine trials, they eliminated two pertinent trials in youth, 382 and 394, that fit their inclusion criteria and were in previous meta-analyses [3]. Neither trial separated from placebo, and in previous meta-analyses [4][5] had the highest levels of suicidal ideation – a significant omission considering the focus of this study. They included the NIMH TADS study [6] and Lilly trials HCJE [7], X065 [8], and LYAQ[9]. LYAQ has been in no other meta-analysis. It was a study of Lilly’s ADHD drug Strattera where the goal was to test the safety of Strattera in cytochrome P450 2D6 poor-metabolizers, the Prozac arm primarily included to see if it affected Strattera levels. Less than 50% were diagnosed with MDD. The baseline mean CDRS-R scores [43.9 and 44.4] barely beat the minimum for inclusion in a MDD study, a third falling below their cutoff score of 36. Including this ADHD trial without identification represents a failure of transparency as well as a fatal flaw in data selection. In addition, there were a number of mathematical errors in the accessible calculations: Table 1. Discrepancies between reported and independent estimates of drug effect.
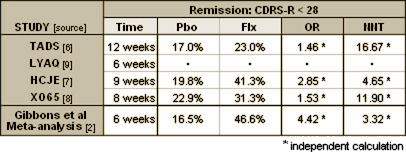
And while the complex regression analysis could not be checked directly, the meta-analysis used the same Remission criteria as the three positive trials in the child cohort [CDRS-R < 28] allowing a comparison of the included trials with the reported effect strength – Odds Ratio and Number Needed to Treat: Table 2. Discrepancies between overall Gibbons meta-analysis estimates
In trial LYAQ, there was no separation from placebo, so the OR would approach 1.0 and the NNT would approach infinity. At only six weeks, this meta-analysis reported a better Remission strength of treatment [higher OR and lower NNT] than any of the trials could achieve even at exit – a mathematical impossibility. How could the composite exceed every individual component? This meta-analysis has been widely publicized as disproving both the FDA “black box” warning about potential suicidality and the questionable efficacy of antidepressants in children with MDD. And yet it is marred by inappropriate data selection kept in the shadows, an opaque methodology, obvious arithmetic errors, and an outcome that was greater than the sum of the parts. This is a flawed study, hardly supporting any broad conclusions about the safety or efficacy of antidepressants in youth. References [1] Gibbons RD, Hur K, Brown CH, Davis JM; Mann JJ; Benefits From Antidepressants: Synthesis of 6-Week Patient-Level Outcomes From Double-blind Placebo-Controlled Randomized Trials of Fluoxetine and Venlafaxine; Arch Gen Psychiatry. Published online March 5, 2012. doi:10.1001/archgenpsychiatry.2011.2044. [2] Gibbons RD, Brown CH, Hur K, Davis JM, Mann JJ; Suicidal Thoughts and Behavior With Antidepressant Treatment: Reanalysis of the Randomized Placebo-Controlled Studies of Fluoxetine and Venlafaxine; Arch Gen Psychiatry. Published on-line February 6, 2012;doi:10.1001/archgenpsychiatry.2011.2048. [3] Clinical Review for NDA 20-151, Supplement SE5-024, Non-Approval Action for Pediatric Supplement for Effexor XR; [online @ http://www.fda.gov/downloads/Drugs/DevelopmentApprovalProcess/DevelopmentResources/UCM163132.pdf#page=2]. [4] Hammad TA, Laughren T, Racoosin J; Suicidality in Pediatric Patients Treated With Antidepressant Drugs; Arch Gen Psychiatry. 2006;63:332-339. [5] Kaizar EE, Greenhouse JB, Seltman H, Kelleher K; Do antidepressants cause suicidality in children? A Bayesian meta-analysis; Clin Trials. 2006;3(2):73-90. [plot online @ http://www.nap.edu/openbook.php?record_id=12829&page=27]. [6] Kennard B, Silva S, Vitiello B, Curry J, Kratochvil C, Simons A, Hughes J, Feeny N, Weller E, Sweeney M, Reinecke M, Pathak S, Ginsburg G, Emslie G, March J; Remission and residual symptoms after short-term treatment in the Treatment of Adolescents with Depression Study (TADS); J Am Acad Child Adolesc Psychiatry. 2006 Dec;45(12):1404-11. [7] Emslie GJ, Heiligenstein JH, Wagner KD, Hoog SL, Ernest DE, Brown E, Nilsson M, Jacobson JG; Fluoxetine for acute treatment of depression in children and adolescents: a placebo-controlled, randomized clinical trial; J Am Acad Child Adolesc Psychiatry. 2002 Oct;41(10):1205-15. [8] Emslie GJ, Rush AJ, Weinberg WA, Kowatch RA, Hughes CW, Carmody T, Rintelmann J; A double-blind, randomized, placebo-controlled trial of fluoxetine in children and adolescents with depression; Arch Gen Psychiatry. 1997 Nov;54(11):1031-7 [9] Kratochvil CJ, Newcorn JH, Arnold LE, Duesenberg D, Emslie GJ, Quintana H, Sarkis EH, Wagner KD, Gao H, Michelson D, Biederman J.; Atomoxetine alone or combined with fluoxetine for treating ADHD with comorbid depressive or anxiety symptoms; J Am Acad Child Adolesc Psychiatry. 2005 Sep;44(9):915-24. |
Great submission Mickey…I’m sure this will be a long wait & see proposition to see if this critique of Gibbons meta-analysis & misleading conclusions will be published or buried deep somewhere…either way, it will be quite interesting to see how this plays out…thank you for your always thoughtful & diligent (if not obsessive) efforts.
The fiduciary duty of a scientific journal and its editorial board requires that substantive errors in published articles be corrected on the record. I do not see how it is possible, therefore, for Archives of General Psychiatry and its editor in chief Joseph Coyle, MD, to deny archival publication of your cogent letter.
Ha! I was just thinking of suggesting that you send it to Ivan Oransky’s Retraction Watch blog. I assume that the Ivan above is one and the same. Gary Schwitzer would also be interested in this, I’m sure.
Have you considered penning an Op-Ed for the NY Times?
Great analysis and wonderful letter. I await its publication.