I’ve seen three or four cases myself and the syndrome is dramatic, though it’s not common. There’s no question that it exists to those of us who have seen it. I expect that most cases don’t even come to medical attention. Someone gets a prescription, starts the medication, and stops it quickly when they start feeling sick – perhaps avoiding that doctor thereafter. I’ve heard many more of those reports in retrospect from patients who have seen other doctors than cases where I’ve been the prescriber. I heard one last week. "Yeah, I tried Prozac. It made me crazy as a f···ing loon. I’ve never felt so suicidal in all my life!"
Correspondence: Fluoxetine and suicide
by David Healy and William Creaney
British Medical Journal. 1991 303:1058.
Sir,Dr Charles M Beasley and colleagues’ analysis of whether there is an association between fluoxetine and suicidality does not entirely settle the question raised by Teicher et al of whether treatment with fluoxetine may in certain instances lead to suicidal ideation. There are several reasons for this.
Firstly, item 3 of the Hamilton scale for depression, ratings on which provide the data for the analysis, is an insensitive measure of suicidality: a rating does not entail the asking of any standard questions, and the anchor points for scoring are not well defined. Furthermore, in interviews characteristic of clinical trials clinicians noting an improvement in depression will tend, by virtue of a halo effect and the counterintuitive nature of the emergence of suicidality in such circumstances, to rate scores down.
Secondly, though Dr Beasley and colleagues’ analysis shows clearly that fluoxetine reduces suicidality in depression, Teicher et al referred to suicidality consequent on the development of symptoms such as akathisia or dysphoria. Dr Beasley and colleagues did not address the question of whether patients taking fluoxetine were more liable to develop akathisia or agitation than patients taking placebo. It seems highly likely that they were. Of these, possibly some may go on to develop suicidal ideation that is in some as yet unspecified way consequent on drug induced akathisia or dysphoria. Such a mechanism seemed to underlie the development of suicidal ideation in two patients taking fluoxetine whom we saw recently.
An alternative method that can be used to determine whether antidepressants lead to the emergence of suicidality entails using a test-retest procedure. There is an ethical problem with such a method in the case of compounds that may induce suicidality. In so far as it was possible to pursue this method we did so; the outcome was a strong suggestion that fluoxetine may lead to suicidality, as may other drugs active on the serotoninergic system (which includes most tricyclic antidepressants).
The significance of the emergence of suicidality during treatment with any psychotropic compounds as opposed to during the course of a depressive episode is that during treatment it can be anticipated and forestalled by warning patients.Though it is helpful to have some of the issues surrounding the question offluoxetine and suicidality tackled so competently, it would be a pity if the rush to douse the flames of media hype surrounding this issue obscured recognition of a phenomenon that the cases originally reported by Teicher et al helped draw attention to.
DAVID HEALY
WILLIAM CREANEY
Academic Subdepartment of Psychological Medicine,
North Wales Hospital, Denbigh, Clwyd
| DEPARTMENT OF HEALTH AND HUMAN SERVICES PUBLIC HEALTH SERVICE FOOD AND DRUG ADMINISTMTION PSYCHOPHARMACOLOGICAL DRUGS ADVISORY COMMITTEE Friday, September 20, 1991 |
||
| P A R T I C I P A N T S | ||
| COMMITTEE: Casey, Daniel, M.D., Chairman Claghorn, James, M.D. Dunner, David, M.D. Escobar, Javier, M.SC., M.D. Lieberman, Jeffry, M.D. Hamer, Robert, Ph.D. Tamminga, Carol, M.D. Lin, Keh-Ming, M.D. Casper, Regina, M.D. Schooler, Nina, Ph.D. |
CONSULTANTS: Hellander, Ida, M.D. Mann, John, M.D. Montgomery, Stewart, M.D. Regier, Darrel, M.D. Stanley, Michael, Ph.D. Teicher, Martin, M.D., Ph.D |
FDA STAFF: Bernstein, Michael, M.P.H. Leber, Paul, M.D. Temple, Robert, M.D. Laughren, Thomas, M.D. Stadel, Bruce, M.D., M.P.H. |
September 20, 1991
…I would suggest to you that I have as little confidence in these anecdotal reports as I do in the anecdotal report of Teicher, and that, in fact, there is no substitute for controlled prospective double-blind clinical trials…
In conclusion, there is simply no scientific evidence whatsoever, no placebo-controlled double-blind study that has established a cause-and-effect relationship between antidepressant pharmacotherapy of any class and suicidal acts or ideation.
As Drs. Potter and Fawcett have suggested, limiting the availability of antidepressants could have a very profound adverse effect in terms of increasing the morbidity and, in fact, mortality associated with untreated depression.
Dr. Charles Nemeroff,
Professor and Chair, Department of Psychiatry,
Emory University, Atlanta Georgia
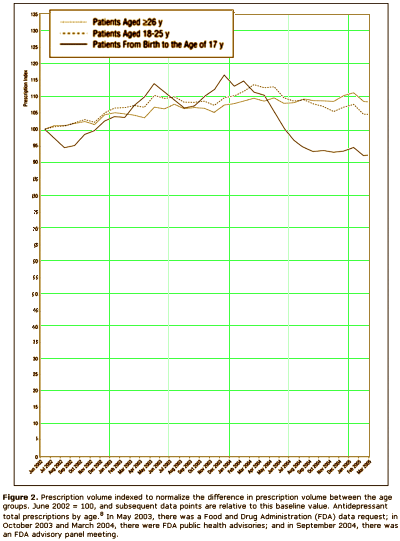
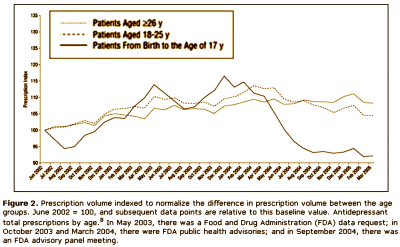
The other group would be the Clinical Trials Group, who would say, "that [we] have as little confidence in these anecdotal reports as [we] do in the anecdotal report of [anybody], and that, in fact, there is no substitute for controlled prospective double-blind clinical trials." On the surface, that appears to be a cry for evidence based medicine. But why do they even care? What difference does it make to them that we would insist on warning [particularly young] patients of the Akasthisia that might occur on antidepressants, even though it’s rare? Why wouldn’t they spend their time doing something more productive? They say that the reason is because by insisting that Akasthisia exists, we are keeping people from prescribing antidepressants to patients who need them to …feel better? …not commit suicide? something like that. So Akathisia doesn’t exist, and they set out to prove its non-existence with statistics and double-blind clinical trials. We Anecdotal Goupies have different hypothesis about why they are so persistent on this point. We think they’re worried about drug sales. This graph is from a Quintiles study [author Dr. Nemeroff] showing a dramatic fall in prescribing antidepressants in kids in response to the FDA warning that was added in 2004 about Akasthisia [the apogee…]:
If you read this blog much, you know I have a habit of redrawing people’s graphs with a real ordinate scale that starts at zero. Here’s why I do that:
So, Dr. Coyle whined about your letter pre-empting space in his journal that would better be devoted to ‘research articles.’ So he consigned it to the non-archival ‘cantina at the end of the galaxy.’ Dr. Coyle needs to recalibrate his editorial and ethical compasses. If Dr. Coyle and his crew at Archives of General Psychiatry had done their jobs right they would have rumbled Dr. Gibbons and his deceits and his errors, and your letter would have been unnecessary. After all, if you as a retired psychiatrist in rural Georgia can do it, why can’t they? They dropped the ball and now they are attempting CYA by prevarication with your letter. As Ivan said in an earlier comment, the journal has a fiduciary duty to correct substantive errors on the record.
Some journals understand this duty, even if Archives of General Psychiatry doesn’t. For instance, Lancet recently published no fewer than 6 critical letters about a misleading stealth infomercial for a novel candidate antidepressant compound (see PubMed ID # 21596429). I think this matter has progressed to the point where Dr. Coyle’s monitor, the editor in chief of JAMA, should be alerted to the ethical issue. No point in relying on Dr. Coyle’s ethical judgment any further.
One quibble on a tangential matter, Dr. Carroll: Dr. Nardo is in no way retired, his protestations to the contrary notwithstanding!
Did everyone read Carl Zimmer’s NYT article about science, research, publication, grantsmanship and retractions? The comments are as important as the article, IMO.
Great series on Gibbons’ obviously skewed research — keep up the good work!