Three months ago, the University of Pennsylvania denied allegations made by one of its professors that several other academics – including his own department chair – allowed their names to be added to a medical journal manuscript, but gave control of the contents to GlaxoSmithKline. As it turns out, a medical communications company called Scientific Therapeutics Information played a large role in preparing the paper, which was published in 2001. The study, which was funded by the drugmaker and the National Institutes of Health, looked at the impact of the Paxil antidepressant on patients with bipolar disorder. The university acknowledged the study was ghostwritten, but did not take any action, because such activity was standard practice between 1998 and 2001, when the study was prepared [back story]. Also known as study 352, it was later published in the American Journal of Psychiatry, although STI was not mentioned…
Double-blind, placebo-controlled comparison of imipramine and paroxetine in the treatment of bipolar depression.
by Nemeroff CB, Evans DL, Gyulai L, Sachs GS, Bowden CL, Gergel IP, Oakes R, Pitts CD.
American Journal of Psychiatry. 2001 158[6]:906-912.
SOURCE: Department of Psychiatry and Behavioral Sciences, Emory University School of Medicine…
OBJECTIVE: This study compared the efficacy and safety of paroxetine and imipramine with that of placebo in the treatment of bipolar depression in adult outpatients stabilized on a regimen of lithium.
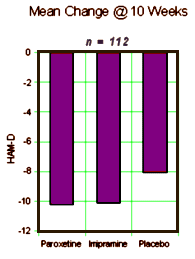
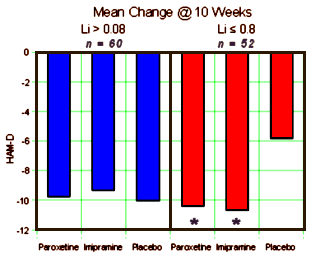
METHOD: In a double-blind, placebo-controlled study, 117 outpatients with DSM-III-R bipolar disorder, depressive phase, were randomly assigned to treatment with paroxetine [N=35], imipramine [N=39], or placebo [N=43] for 10 weeks. In addition to lithium monotherapy, patients may have received either carbamazepine or valproate in combination with lithium for control of manic symptoms. Patients were stratified on the basis of trough serum lithium levels determined at the screening visit (high: >0.8 meq/liter; low: < 0.8 meq/liter). Primary efficacy was assessed by change from baseline in scores on the Hamilton Rating Scale for Depression and the Clinical Global Impression illness severity scale.
RESULTS: Differences in overall efficacy among the three groups were not statistically significant. For patients with high serum lithium levels, antidepressant response at endpoint also did not significantly differ from placebo. However, both paroxetine and imipramine were superior to placebo for patients with low serum lithium levels. Compared to imipramine, paroxetine resulted in a lower incidence of adverse events, most notably emergence of manic symptoms.
CONCLUSIONS: Antidepressants may not be useful adjunctive therapy for bipolar depressed patients with high serum lithium levels. However, antidepressant therapy may be beneficial for patients who cannot tolerate high serum lithium levels or who have symptoms that are refractory to the antidepressant effects of lithium.
The University of Pennsylvania has denied allegations made by one of its professors that several other academics – including his department chair – allowed their names to be added to a medical journal manuscript, but gave control of the contents to GlaxoSmithKline, according to his attorney. The study, which was funded by the drugmaker and the National Institutes of Health, looked at the impact of the Paxil antidepressant on patients with bipolar disorder.At the same time, the university has acknowledged a claim by the professor, Jay Amsterdam, that the 2001 study was ghostwritten by Scientific Therapeutics Information, his attorney tells us. However, he says the university is not planning on taking any action in connection with the ghostwriting. The study, which was published by the American Journal of Psychiatry [see here], did not mention that STI played any role [here is an email in which STI employee Sally Laden discusses that she would work on the paper].
“They said his allegations were not meritorius, although they did find that the publication at issue was ghostwritten,” says Bijan Esfandiari, the attorney, citing a letter and other documents he received from the university. “They acknowledged that a marketing firm was involved in drafting, and everything associated with, the issue. But in response to our complaint, they said that, at the time these events took place, which was between 1998 and 2001, ghostwriting was standard practice and everyone was doing this, so therefore, we’re not going to punish any individuals”…
…Having filed a complaint about scientific misconduct a year ago with the federal Office of Research Integrity, Amsterdam is now charging that the university “went out of its way to find a path to whitewash the conduct of its own employees,” instead of admonishing his colleagues for allowing their names to be appended to a manuscript that was drafted and revised by a ghostwriter, according to a letter sent yesterday to the ORI…In attempting to debunk the recent UPenn investigation, Amsterdam notes the committee contended that any study flaws, such as insufficient sample size that resulted in an underpowered study, was the responsbility of a Rosemary Oakes, a Glaxo biostatistician and a named author. But he maintains that every study author had equal responsibility to ensure the final paper was accurate. The committee, meanwhile, did not interview Oakes, according to the letter sent to ORI.
“The evidence appears to indicate that the academic authors were almost completely uninvolved in the data analysis and manuscript preparation, and were merely serving as key opinion leaders on the manuscript for commercial and marketing purposes,” the letter states. There is more. The letter goes on to say that, while Jack Gorman was serving as editor of the American Journal of Psychiatry and “closely involved in the editorial review process of the 352 study,” he was also on the Glaxo speaker bureau and served as a consultant to Glaxo for promoting Paxil, “including participation in GSK-funded symposia, advertising videos, GSK-funded lectures, co-authoring GSK-funded articles in medical journals and book chapters.”
Supported by NIMH grant MH-51761 and a grant from GlaxoSmithKline.
Sorry, the comment form is closed at this time.