A CONTROLLED TRIAL OF IMIPRAMINE IN TREATMENT OF DEPRESSIVE STATES
BY J. R. B. BALL AND L. G. KILOH
British Medical Journal. 1959 2:5199:1052-1055.
[full text on-line]
All the cases included in the trial were out-patients, and anyone showing gross retardation or extreme agitation and those in whom the risk of suicide was considerable were automatically excluded, as they were admitted for in-patient treatment. Nevertheless, a number of the patients included were quite severely depressed. One did commit suicide during the trial shortly after starting on the placebo. He was adamant in his refusal to come into hospital. The clinical trial was organized in conjunction with the hospital dispensary, and cases were allocated at random to the active and placebo groups by the hospital pharmacist. On the first and second days of treatment four 25-mg. tablets were given ; the dosage was increased to six, eight, and finally ten tablets daily by the fifth day. Patients were seen weekly, and this dosage was maintained for four weeks. If no improvement had occurred at the end of this period the drug was regarded as ineffective and some other form of treatment was instituted. In most cases the alternative tablets were given for a further period. The results of this crossover trial are not yet available and will be reported at a later date. Patients who responded to the tablets they were receiving were kept on these until maximal improvement was obtained. Without exception this occurred within six weeks of starting treatment. How long it is necessary to continue the administration of imipramine to avoid the risk of relapse has not yet been established…The results of treatment were assessed as symptom free, greatly improved, somewhat improved, no change, or worse. For the purpose of assessing the value of the drug as a significant therapeutic agent, the first two of these categories have been combined as a good or worth-while result and the other three as a poor result. Patients showing a good result were able to return to their normal activities without undue effort…
Of 55 cases of endogenous depression treated, 27 received imipramine and 28 the placebo. On imipramine 20 responded well, while 7 did badly. On the placebo, 6 did well and 22 badly. This is a highly significant difference, and, with Yates’s correction applied, P<0.01. Of the 42 cases of reactive depression 22 received imipramine and 20 the placebo. Of those given imipramine 13 were improved and 9 were not; while of the 20 controls 4 improved and 16 showed a poor result. This, too, is a significant result (P<0.02). When improvement occurred it was nearly always evident by the end of the third week, sometimes as early as the fourth or fifth day. The mean was 9 to 10 days. Of the 27 cases of endogenous depression treated with imipramine 20 were females and 7 were males. Of the 20 women 14 did well, and of the 7 men 6 had a good result. Of the 22 cases of reactive depression given imipramine 9 out of 14 women did well, compared with 4 out of 8 men. Freyhan (1959) has suggested that women respond better to imipramine than men. These figures lend no support to this view…
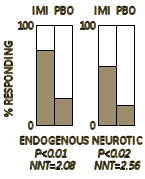 In an earlier time, clinical trials were done by psychiatrists on their own patients using the manifest clinical response. This was a four week trial that established the efficacy of Imipramine in depression. The outcome variable was "symptom free or greatly improved." As shown on the right, the response was highly significant in both endogenous and neurotic depression, with a number needed to treat value between 2 and 3. There was no need for rating scales. Even the statistics were window dressing for the obvious. All you really need to see is the graph to know that the medication worked. They did not think the medication was a euphoric but rather had a specific effect on depression itself – hoping that further study would lead to an understanding of the basic mechanisms of depression.
In an earlier time, clinical trials were done by psychiatrists on their own patients using the manifest clinical response. This was a four week trial that established the efficacy of Imipramine in depression. The outcome variable was "symptom free or greatly improved." As shown on the right, the response was highly significant in both endogenous and neurotic depression, with a number needed to treat value between 2 and 3. There was no need for rating scales. Even the statistics were window dressing for the obvious. All you really need to see is the graph to know that the medication worked. They did not think the medication was a euphoric but rather had a specific effect on depression itself – hoping that further study would lead to an understanding of the basic mechanisms of depression.The real importance of these new antidepressive drugs appears to be twofold. Firstly, they provide an effective, easily administered, and readily acceptable form of treatment in most cases of depressive illness, which is one of the most important categories of mental disturbance with which the physician has to deal. Secondly, the modes of response to these drugs indicate lines of research which may well lead to the elucidation of the aetiological factors which play an immediate part in determining the onset of depressive illness. One feels hopeful that a study of the metabolism of these drugs and of their effect on the brain enzyme systems may well have far-reaching results, for it appears likely that they act upon what one might term the basic mechanisms in depression, and they are certainly more than euphorizing agents like the amphetamines.
We thank our colleagues at the Royal Victoria Infirmary and at the Newcastle General Hospital for allowing us to include their patients in this clinical trial, and we are pleased to acknowledge our indebtedness to Professor Martin Roth for his advice and encouragement. We are grateful to Geigy Pharmaceutical Company Ltd., who supplied the imipramine ("tofranil") and placebo tablets, and particularly to Dr. Alan Broadhurst for his valuable co-operation.





It was such a more innocent time. I appreciated particularly the comment by Dr. Broadhurst, "Like so many other discoveries in pharmacology, serendipity had played a major role. The antidepressant effect of imipramine was completely unexpected and was discovered entirely by accident. Luck was on my side too. I just happened to be working in the right place at the right time." That is, of course, the way medical discoveries usually happen. Astute observers working on one thing notice something else altogether and have the wisdom to follow their noses – a bit of mold in an old perti dish. So unlike the forced marches proposed by Dr. Insel and friends determined to wring out scientific discovery as in just plain crazy!…, the scientists involved were not constrained by the dictates of Translational Medicine or Requests for Applications [RFA]. The scientists were the people actually doing the research and evaluating the patients, not KOLs writing review articles, whining about an Empty Pipeline, amassing CVs with hundreds of authorship cites, awaiting the data on paid volunteers from commercial clinical research organizations, or collecting honoraria under the table…

Thank you for pulling this story together, Mickey. When I re-read the 1959 paper by Ball and Kiloh on the efficacy of imipramine, the effect is like being present at the creation. You reflected on the credibility of data obtained in contemporary clinical trials. The problem you flag is what we call ecological validity. In Ball and Kiloh (1959), ecological validity is reinforced by this excerpt: It has certainly been obvious in this department that since the introduction of iproniazid and imipramine the amount of in-patient and out-patient E.C.T. given has fallen sharply. Now that’s what I call ecological validity – they didn’t need to cherry pick primary and secondary endpoints as in Glaxo Study 329 courtesy of Sally Laden at STI on behalf of investigators who had only the most cursory knowledge of the patients and on behalf of a corporation that called the shots for the sake of marketing.
Colleagues
Does arouse nostalgia. When we did our imipramine and mepazine studies–as I recall our contact with industry was to request drug which they freely sent with no requests but the implicit scientific attitude that we should communicate to the public whatever we found. These included the findings that mepazine did not work,the antidepressant effect of chlorpromazine, the serendipitous discovery of panic disorder, and its Imipramine treatment as well as the first report of a non-study sudden death in a child treated clinically with high dose Imipramine. I could continue but it seems clear that NIMH and industry are self-deluded. Yearning for clinical serendipity seems fruitless but if we had a good computerized post-marketing system, computerized serendipity is possible. Ridiculous ??–see Finnish study of bleeding episodes associated with serotonergic anti-depressants which also noted their beneficial prophylaxis regarding myocardial infarction —via computerized hospital records.
Don Klein
Thank you for posting this and thank you for your excellent work. I would still be interested int he outcome if an active placebo had been added. As compelling as these findings are, there is no question that everyone in the study would have had a good idea of who was getting active drug. This could have influenced out come, not in the cynical way we observe today, but in a way that is just part of human nature.