 Since reviewing the study by Ball and Kiloh that validated the efficacy of Imipramine in 1959 [remembrance of things past…], I’ve had a nagging disquiet about our ways of determining the efficacy of psychoactive medications in general and the antidepressants in the specific. In that classic study, the 97 subjects were help-seeking patients and the response was measured by a clinician interviewer assessing the reported improvement by the subjects. I doubt any of us would question their conclusion that Imipramine was an effective treatment for depression. I certainly didn’t. In that study, each piece along the way was subjective – from initial diagnosis to drug treatment treatment response.
Since reviewing the study by Ball and Kiloh that validated the efficacy of Imipramine in 1959 [remembrance of things past…], I’ve had a nagging disquiet about our ways of determining the efficacy of psychoactive medications in general and the antidepressants in the specific. In that classic study, the 97 subjects were help-seeking patients and the response was measured by a clinician interviewer assessing the reported improvement by the subjects. I doubt any of us would question their conclusion that Imipramine was an effective treatment for depression. I certainly didn’t. In that study, each piece along the way was subjective – from initial diagnosis to drug treatment treatment response.

A double-blind comparison of fluoxetine, imipramine and placebo in outpatients with major depression
by Feighner JP, Boyer WF, Merideth CH, Hendrickson GG.
International Clinical Psychopharmacology, 1989 4[2]:127-134.
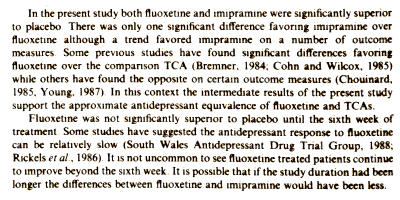
Fluoxetine is the first selective serotonin reuptake inhibitor antidepressant to be marketed in the U.S. In this double-blind trial fluoxetine was compared with imipramine and placebo among 198 outpatients with DSM-III major depression, of whom 145 completed at least 2 weeks of active treatment and were evaluated for efficacy. Significantly fewer patients in each active drug group terminated early due to lack of efficacy compared to placebo. Both imipramine and fluoxetine were significantly superior to placebo on most measures. There were no consistently significant differences between the two active drugs although a trend favored imipramine on a number of measures. Fluoxetine was generally well tolerated. Significantly more imipramine than placebo patients terminated early due to side-effects while the fluoxetine-placebo difference was not significant. The results support previous studies which suggest fluoxetine’s superior side-effect profile and the approximate antidepressant equivalence of fluoxetine and TCAs.
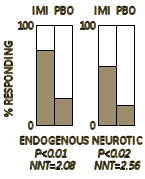
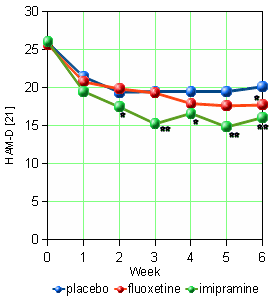 This study was of 198 recruited subjects diagnosed with Major Depressive Disorder by DSM-III criteria and followed using the Hamilton Depression Rating Scale [21 items] administered by four physician raters at weekly intervals [authors?]. Subjects were required to have a HAM-D score > 20 after a variable washout period for inclusion. There were many dropouts [Pl 66%, Flx 51%, Imi 48%] corrected using the LOCF method. So at the dawn of the Prozac era, this clinical trial had been objectified, but it had the now familiar problem of high drop-out rates requiring mathematical correction. Neither clinical response nor remission were defined, relying on significant separation from placebo as the outcome parameter. There were no conflict of interest declarations and the funding source was not specified, though this was an Eli Lilly funded study.
This study was of 198 recruited subjects diagnosed with Major Depressive Disorder by DSM-III criteria and followed using the Hamilton Depression Rating Scale [21 items] administered by four physician raters at weekly intervals [authors?]. Subjects were required to have a HAM-D score > 20 after a variable washout period for inclusion. There were many dropouts [Pl 66%, Flx 51%, Imi 48%] corrected using the LOCF method. So at the dawn of the Prozac era, this clinical trial had been objectified, but it had the now familiar problem of high drop-out rates requiring mathematical correction. Neither clinical response nor remission were defined, relying on significant separation from placebo as the outcome parameter. There were no conflict of interest declarations and the funding source was not specified, though this was an Eli Lilly funded study.The graph shown here is drawn from a table in the paper using a full ordinate scale. It hardly supports the statement in the abstract, "…the approximate antidepressant equivalence of fluoxetine and TCAs" echoed in the full text below:
This post hasn’t gone as planned. I was going to look at studies around the time of Prozac’s entry to the market [1987] and recently that had Imipramine as an active comparator to see if its potency waned, suspecting that it would as a function of patient selection [recruits]. I also was vaguely thinking that the objectification and impersonality of modern studies might be a factor to look at. But once I looked at Feighner’s 1989 study, I took another turn. It was written with a lot of ‘spin,’ even in 1989. I was surprised that it started that early. I guess I thought that the PHARMA colored articles developed later, but there it was as Prozac came out of the gate. I was surprised that there was zero conflict of interest information, and that this obviously Lilly funded study had zero acknowledgement of that fact. I redrew their graph and it didn’t look so hot for Prozac to me. In the abstract, they said, "Significantly more imipramine than placebo patients terminated early due to side-effects while the fluoxetine-placebo difference was not significant" which was true, but that was off-set by Prozac drop-outs due to lack of efficacy [Pl 66%, Flx 51%, Imi 48%].
Fluoxetine versus other types of pharmacotherapy for depression
by Cipriani A, Brambilla P, Furukawa T, Geddes J, Gregis M, Hotopf M, Malvini L, and Barbui C.
Cochrane Database Systematic Reviews. 2005 19(4):CD004185.
AUTHORS’ CONCLUSIONS: There are statistically significant differences in terms of efficacy and tolerability between fluoxetine and certain ADs, but the clinical meaning of these differences is uncertain, and no definitive implications for clinical practice can be drawn. From a clinical point of view the analysis of antidepressants’ safety profile (adverse effect and suicide risk) remains of crucial importance and more reliable data about these outcomes are needed. Waiting for more robust evidence, treatment decisions should be based on considerations of clinical history, drug toxicity, patient acceptability, and cost. We need for large, pragmatic trials, enrolling heterogeneous populations of patients with depression to generate clinically relevant information on the benefits and harms of competitive pharmacological options. A meta-analysis of individual patient data from the randomised trials is clearly necessary.
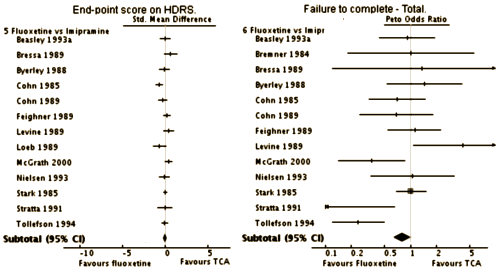
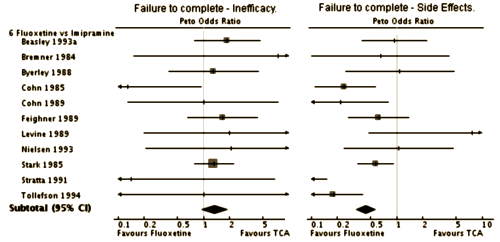
As you can see, it was pretty much a wash on efficacy – no real differences when they compared the studies. There was a small weighting towards Prozac when it came to drop-outs. They broke down the reasons for the drop-outs into Lack of Efficacy and Side Effects:
As a nation, we need to do a better job of financially supporting scientific research, especially on the University level. Open source policies for research may also have a sanitizing effect on research. It’s sad that we’ve learned to accept one unreplicated study as proof of anything and that are our scientific journals are as lame as they are.
“Free-market capitalism” and scientific research are lousy bedfellows. Most journalists are laughably bad conveyors of scientific news. The direct to consumer drug advertising and astroturfed groups promoting drugs is shameful.
Drug research needs to be public property.
Oh yeah, Cochrane. Poor things have to make do with whatever studies they can find. For psychiatry, it’s GIGO.
Per Cochrane review: “From a clinical point of view the analysis of antidepressants’ safety profile (adverse effect and suicide risk) remains of crucial importance and more reliable data about these outcomes are needed. Waiting for more robust evidence, treatment decisions should be based on considerations of clinical history, drug toxicity, patient acceptability, and cost.”
Without that “reliable data,” which is still forthcoming (see David Healy’s latest posts on antidepressants and pregnancy http://davidhealy.org/blog/ ), clinicians cannot assess “drug toxicity.” They’re still prescribing as though these drugs have negligible risk and ignoring presentations of adverse effects when they occur.