Out of curiosity, I looked back using the Healthy Skepticism Archive to see the timeline of Study 329. The proposal to do the study is dated December 5, 1992, and the study protocol is dated April 17, 1994. The study itself went on from 1994-1998 – a comment from the first pass at the results [October 14, 1998]:

But by then, Scientific Therapeutic Information [Sally Laden] had made a proposal to write a journal article [April 3, 1998], which was well on its way by February 1999 [letter from Martin Keller, P.I. and senior author to Sally Laden, ghost-writer at STI]:

It was initially rejected by the JAMA, then finally accepted by the JAACAP on February 15, 2001 and published in the July 2001 issue. Among the suits filed about this paper, one filed by Eliot Spitzer in New York in 2004 was settled out of court – requiring GSK to set up a Clinical Trials Registry. The one they set up was very much like the one on the left in the first figure above. It did not have the raw data itself.
New Topic:
In the fall of 2009, at the height of fears over swine flu, our research group discovered that a majority of clinical trial data for the anti-influenza drug Tamiflu — data that proved, according to its manufacturer, that the drug reduced the risk of hospitalization, serious complications and transmission — were missing, unpublished and inaccessible to the research community. From what we could tell from the limited clinical data that had been published in medical journals, the country’s most widely used and heavily stockpiled influenza drug appeared no more effective than aspirin. After we published this finding in the British Medical Journal at the end of that year, Tamiflu’s manufacturer, Roche, announced that it would release internal reports to back up its claims that the drug was effective in reducing the complications of influenza. Roche promised access to data from 10 clinical trials, 8 of which had not been published a decade after completion, representing more than 4,000 patients from every continent except Antarctica. Independent verification of the data seemed imminent. But more than two years later, and despite repeated requests, we have yet to receive even a single full trial report. Instead, the manufacturer released portions of the reports, most likely a very small percentage of the total pages. [One of us, Tom Jefferson, has been retained as an expert witness in a lawsuit relating to some of these issues].This is entirely within Roche’s rights. After all, regulators have never required drug or medical device manufacturers to share their data with independent researchers or academics. They are required to show the information only to the regulators themselves, who treat the data as secret. Some may argue that, because the Food and Drug Administration approves drugs for the United States market based on these data, this is not a major cause for concern. But the actual use of drugs is often driven by assumptions about drug safety and effectiveness put forth by articles in peer-reviewed journals [sometimes written by doctors affiliated with the drug manufacturers] and clinical practice guidelines that can be entirely inconsistent with the F.D.A.’s assessments…
The only agency in the United States that seems to have independently reviewed the original trial data never made these claims. The F.D.A.’s conclusion — which it required Roche to print on Tamiflu’s product labeling — is that “Tamiflu has not been shown to prevent” complications like serious bacterial infections [for instance, pneumonia]. It seems that federal agencies like the C.D.C. and H.H.S., instead of conducting an independent evaluation of Tamiflu, advocated stockpiling by referencing claims in journal publications written by the drug’s manufacturer, ignoring the F.D.A.’s assessment that those very claims were unproven. Why would they do this? Unwarranted trust in the peer-review process of medical journals probably has something to do with it. So, too, does wishful thinking; lacking good alternatives, it’s tempting to hope that the drug we have works wonders. And it’s important to remember that correcting the statements of medical journals or public health agencies falls outside the F.D.A.’s jurisdiction — when it comes to drugs, the F.D.A. is responsible for regulating industry, not other government agencies…
Nevertheless, the data point to a drug of minimal benefit. In accordance with the F.D.A.’s findings, it appears to shave a day off the duration of influenza symptoms, but we found no decrease in risk of hospitalization and no evidence that it could stop the spread of the virus. More worrisome, we found suggestive evidence that Tamiflu interfered with the body’s ability to produce antibodies against influenza — which could affect the body’s response to influenza vaccine and its ability to fight off future influenza infections. But to do a complete analysis, including evaluating Tamiflu’s potential harms, we need the remainder of the data — the full “clinical study report” — promised by Roche, but never delivered.
In response to our conclusions, which we published in January, the C.D.C. defended its stance by once again pointing to Roche’s analyses. This is not the way medical science should progress. Data secrecy is a disservice to those who volunteer their bodies for clinical trials, and is dangerous to those being asked to swallow approved medicines. Governments need to become better stewards of the scientific process. The European regulator’s announced intention to release clinical study reports after it finishes reviewing a manufacturer’s application is an important precedent. But the F.D.A. — guardian of arguably more trial data than any other entity in the world — appears stuck in the era of data secrecy. We should not have to wait for patients to be hurt by the medications they take, as recently happened with the diabetes drug Avandia, before reviewing this wealth of data.
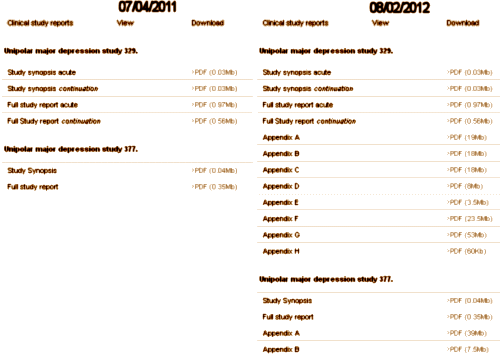
So, back to Paxil Study 329. Peter Doshi noticed that the raw clinical data from GSK’s Paxil Study 329 was missing from the web site above [Paroxetine and pediatric and adolescent patients] and questioned whether it was fulfilling the settlement requirements from the old 2004 NY GSK suit. I don’t know the details of how it came to be, but here on August 2, 2012, the data has finally been posted [after another $3B suit just settled against GSK also related to this study] – 11 years after the publication of the article and 8 years after data transparency was prescribed by the New York settlement. Better late than never I suppose, but way earlier would have been infinitely preferable.
We owe a debt of gratitude to Peter Doshi, Tom Jefferson, and their colleagues for going beyond their frustration with the lack of available data to vet the Tamiflu data, and taking on the broader issue of the secrecy surrounding the pharmaceutical clinical trials in general. When I started looking at the clinical trials for the psychiatric drugs several years ago, I spent hours poring through FDA records finding very little raw data – mostly summaries. At least that proved to be more informative than the published papers, but it was all second-hand stuff. The clinical trial information has been held closely as if it’s a proprietary secret like the formula for Coca Cola. It’s not. It’s rather scientific data to be vetted by whomever because it has everything to do with the medications we prescribe to patients. These authors make their case very well themselves in this publicly available article in PLoS Medicine, and I recommend it as first rate – hopefully the beginning of a movement:
Dr. Healy has boiled this down very simply to: Full disclosure of all clinical data=SCIENCE; current practices of releasing summaries, tables, etc… is NOT science.
Pharmaceutical companies cannot claim exclusive rights/ownership of the data they collect via their paid academic medicine researchers, AND claim to be a business that provides products to treat symptoms and illnesses that have to be diagnosed by physicians. Without respect for the scientific process that is (or should be) the foundation for the practice of medicine; they are not qualified to be a VENDOR…
Consider: Doctors are not shopping Chinese herbal catelogs; homeopathic web sites, to determine which remedy will be best for their patients. And to think we are told in a very condescending, patronizing way that this would be absurd! Why? THESE natural substances have not met rigorous scientific standards to be considered *treatments for diagnosed illnesses*.
Many years ago, Pharma lobbied Congress to stipulate that data generated in clinical trials are proprietary secrets, and the FDA has been obliged to respect that ruling ever since. Having a clinical trials register like ClinicalTrials.gov hasn’t helped promote independent access to patient level data – that’s what it would take for independent analyses of corporate claims.
Seems to me it is time to declare that clinical trials are too important to be left to the corporations. This means it is time to change the law. The present system exploits the altruism of patient volunteers in clinical trials to the pecuniary advantage of the corporations. It allows the corporations to bury unfavorable trials and to manipulate the data in other ways – as we now know from Glaxo Study 329. In other words, the corporations treat patient volunteers as commodities instead of as partners in a principled research enterprise.
A change in the law would greatly reduce the number of experimercials that are conducted – these are pretend clinical trials that have a marketing goal rather than a genuine scientific objective. There are many of these phony exercises, often conducted at academic medical centers, which end up diverting the available clinical research infrastructure away from worthwhile studies.
Makes you wonder why doctors would continue to prescribe drugs that have been developed and tested in a shroud of malfeasant secrecy.
THESE natural substances have not met rigorous scientific standards to be considered *treatments for diagnosed illnesses*.
Natural substances cannot be patented (yet), thus there is no profit motive. Isn’t it amazing that “marijuana has no medical benefit” but synthetic marijuana does? There’s no money to be made with placebo-controlled, double blind studies of natural substances . . . thus no money will be expended testing them.
Profit motive may be operating when we consider:
There’s no money to be made with placebo-controlled, double blind studies of natural substances . . . thus no money will be expended testing them.
But what accounts for classifying natural substances as *non-treatment* and thus not reimbursed by our health care plan? Considering it is an act of consciously motivated omission that renders them *untested* , how do we get from Not worth testing, to Not an option for health care insurance consumers?
By most accounts, synthetic marijuana is very subpar in terms of medicinal benefits. Of course there will be no federally controlled testing of this anecdotal evidence.