| NOTE: |
| "Out of curiosity, I looked back using the Healthy Skepticism Archive to see the timeline of Study 329. The proposal to do the study is dated December 5, 1992, and the study protocol is dated April 17, 1994. The study itself went on from 1994-1998 – a comment from the first pass at the results [October 14, 1998]:"
The links in red from an earlier post [a movement…] aren’t working today for some reason. I had already clipped the parts that introduce this post so they’re here unlinked for the moment… |

In the SKB [GSK] memo I ended with, it goes on to say…

which actually makes little sense. I guess the Study 329 just wasn’t negative enough [degrees of negativity?]. So it became a poster at the October 1998 European Congress of Neuropsychopharmacology [Ray Berard and Neal Ryan]. In that presentation, the failure to separate from placebo wasn’t mentioned directly, instead it said:
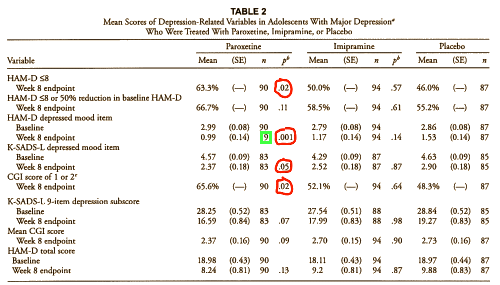
This idea of trends became the watchword thereafter – that and some secondary measures which were to later show up in the Keller et al 2001 paper in this table:
But while the posters and the paper settled for "effective treatment," at Corporate [SKB/GSK] there was more energy:
The drift from "did not reach statistical significance in the primary endpoints," to "effective treatment for major depression in adolescents," to "generally well tolerated and effective for major depression in adolescents" [Keller et al], to "cutting edge" and "REMARKABLE Efficacy and Safety in the treatment of adolescent depression" appears to have been seamless. It just happened.
And while this infectious enthusiasm rippled through SKB [now GSK] and among the authors, the FDA wasn’t impressed. As decided earlier, SKB [GSK] didn’t submit it for a pediatric approval, but it was submitted as part of a package in 2002 for a pediatric extension for exclusivity. The FDA said:




In the report of Study 329 in JAACAP there is a glaring deceit. It is in Table 2, which you reproduced above. Table 2 summarizes evidence of efficacy but it glosses over the predefined, primary efficacy measures. These were “The protocol described two primary outcome measures: (1) response, which was defined as a HAM-D score of 8 or a 50% reduction in baseline HAM-D score at the end of treatment; and (2) change from baseline in HAM-D total score.” The first of these primary outcomes measures is the second row in the Table, but it is not identified there as a primary outcome measure, and it was nonsignificant! The second primary outcome measure vanished from the manuscript. Presto fixo! And these people expect to be taken seriously?