I’ve always wondered how Dr. Martin Keller, then the recently named Chairman of Psychiatry at Brown [1989], got involved in a study of Paxil in adolescents. He’s neither a Child nor Adolescent psychiatrist. Paxil had been approved for MDD in adults by the FDA in December 1992, and the proposal for what would become Study 329 in adolescents was also dated December 1992. When it was approved for funding by SKB in 1993, Dr. Neal Ryan, a Child Psychiatrist at Pittsburgh, was the Principal Investigator. There was another now-familiar name associated with the early days of Paxil, Dr. Charles Nemeroff. In 1991, Dr. Nemeroff became Chairman at Emory. That year, he had testified as an expert for Eli Lilly at an FDA hearing about Prozac and suicidal thinking [he said it didn’t happen]. In 1993, Sally Laden of STI [who would later ghost-write the Study 329 paper] organized SKF’s Paxil Advisory Board to meet in November 1993 and chose Dr. Nemeroff as moderator. The main focus of the meeting was on getting Primary Care Physicians to treat depression [with Paxil]. Sally Laden’s STI would later ghost-write Drs. Nemeroff’s and Schatzberg’s 1999 textbook for Primary Care heavily weighted towards Paxil [come to think of it, Dr. Schatzberg also became Chairman at Stanford in 1991]. So SKF’s launch of Paxil had collected a number of the coming KOL class [not to mention a who’s who of young academic Child Psychiatrists involved in Study 329 itself]. It was the dawn of a new era, the NIMH decade of the brain, and the race was on to include pediatric depression in the pantheon of indications for the SSRIs [tuning the quartet…].
| My apologies up front, but I’m about to go off on a tangent… |
| By 1998, the data from the Paroxetine Trials was in and they hadn’t fared very well. Study 377, a non-US companion to Study 329 was a bust, and the best they could say about 329 was that it showed trends. They decided not to go for FDA approval, but present the 329 data as a poster at the European College of Neuropsychopharmacology, and to develop it as a Journal article using Scientific Therapeutics Information with Sally Laden as the writer. So the first question is, how did they decide to ignore the study’s failure to meet either primary outcome parameter and simply lump the outcomes into a long list? Here’s one answer from the horse’s mouth [Deposition of Sally Laden, 2007]:
QUESTION: Okay. This provided you with information that you then utilized to prepare the first draft of the manuscript for Study 329?
ANSWER: Yes QUESTION: Okay. Was it your responsibility alone to create the first draft of Study 329 or did you get help from some of your colleagues? ANSWER: I believe I created it on my own. QUESTION: Okay. Did Martin Keller tell you what to put in the first draft? ANSWER: I don’t recall. I don t think I had any conversation with him until we were, you know, afterwards. QUESTION: Okay. After you prepared the first manuscript? ANSWER: To the best of my recollection, yes. … QUESTION: In here you list eight main outcome measures, correct? ANSWER: Yes QUESTION: And you can’t tell from reading – a reader could not distinguish which are these – whether any or all of them are primary or secondary? ANSWER: Correct QUESTION: Okay. My question was do you know whose idea it was to not distinguish between primary and secondary efficacy measures? ANSWER: A reader cannot. This was a first draft, so this came straight from me. This was, I guess, my interpretation. I m remembering this may have been my interpretation of the data. It’s hard to get the time sequence of these early pieces straight, and maybe it doesn’t matter so much as I think it does. The SKB[GSK] position paper announcing the ECNP poster and the article is dated October 14, 1998, but the copy of the poster is also dated "OCT ’98" [from GSK]. The poster is by Dr. Ray Berard, the PI for failed Study 377 and Dr. Neal Ryan, the PI of Study 329. They took a different tack from Sally. They stated the Primary Efficacy Parameters clearly in the Methods:
• the proportion of responders with a >50% reduction in the total HAM-D score or a HAM-D score of <8 at week 8.
• change in baseline in HAM-D total score at week 8.
But in the Results, they say:
Primary Efficacy Parameters:
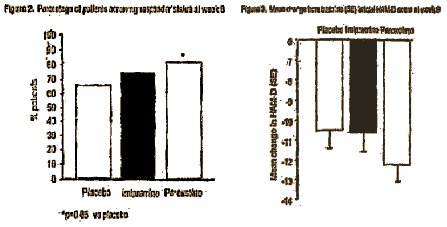
• By week 4, approximately half the patients in each treatment group were classified as responders (Figure 2). This proportion increased further during the study and by week 8 there was a statistically significant greater number of responders in the paroxetine group compared with placebo (81% vs 65%). The Imipramine group did not separate from placebo for this efficacy parameter (73% vs 65%).
• After 8 weeks of treatment, patients receiving paroxetine experienced a greater decrease in total HAM-D score than the placebo group (Figure 3). The difference showed a trend towards statistical significance. The change in HAM-D score in the Imipramine group was comparable with that of placebo.
One has to wonder where the numbers in red above came from. That’s not what I [and others] found from the raw data, nor is it what was reported in the article [see Table 2].
|
| End of tangent |
We’re so used to how clinical trials have been handled in psychiatry over the last couple of decades that the goings on here in the period after the results were in from Study 329 don’t seem particularly odd. But they’re plenty odd. The people making decisions are at the pharmaceutical corporate headquarters rather than in the offices of the academics supposedly doing the study. As they say in an October 1998 position paper, the first order of business is whether they can submit their studies to the FDA for approval [sell Paxil as a treatment for depression in adolescents]. They consult with "Regulatory" and "Marketing" and the answer is obviously "no" – "unacceptable commercially":

So what’s next? A poster and an article – apparently already in the works:

What’s the point of that? To support "off-label" promotion and sales is the only thing I can think of. I don’t know who made the poster, but my guess would be STI. The sponsors were the PIs from the two studies [377 and 329]. And Sally Laden got to work on the article. Who made the decision about how to slant the data in the paper and poster? Sally says she thought it up all by herself. I doubt that. I expect Jim McCafferty was involved, as he seems to be SKB’s man in charge. He sent her the study summary. I doubt the authors, and principle investigators were much involved. I don’t say that to exonerate them but rather as an indictment. It looks like the Study 329 leader, Dr. Martin Keller, didn’t really come to the party until after the paper had been written in draft form – until after Sally had shuffled the outcome variables. I wonder if he noticed. And the only thing he had to say was this [famously] to ghost-writer Sally:
I think this is the picture I have in my mind’s eye of a large subgroup of psychiatrist/scientists in the current KOL generation, sitting at their desks hearing about the clinical trials where they’re listed as Principal Investigator [and/or Author] by a call or email from the pharmaceutical company actually doing the study [or the ghost-writer-in-charge] to fill them in on how things are progressing, what the poster or the paper is going to look like. I wonder how many on the author list met the patients in Study 329? or their parents? knew their names? took care of them in the hospital when they overdosed? dropped by the clinic to see how things were going? I’ll bet there weren’t many. I expect these patients were seen by assistants. I wonder how many of the authors drew a graph? or made a table? or checked the figures for accuracy? I expect that was all done by STI and GSK. Had any of them seen the kids who became hostile to know if that was out of character – like maybe an idiosyncratic drug effect? Did any author know these kids any better than I do looking at the computer readouts? I really doubt it…
So, the first lesson I would take from Study 329, a Clinical Trial, is that there’s no evidence that the authors were clinicians, and by extension, they were not clinical scientists. They don’t really appear in the story until the results were in, the pharmaceutical company had chosen its directions, the presentation format was chosen, and the work towards publication was already well in progress. Maybe they consulted along the way, but they certainly were not the author-ity. And while it’s possible that they were involved at their own sites, I expect it was administrative rather than clinical involvement. If I’m wrong about that, the ball’s in their court to prove it. It sure doesn’t show.
ANSWER: I don’t recall. I don’t think I had any conversation with him until we were, you know, afterwards.
QUESTION: Okay. After you prepared the first manuscript?
ANSWER: To the best of my recollection, yes.
If you’ve read the comments on the blog recently, you’ll know that I’m being encouraged to focus this story on the foibles of the American Academy of Child and Adolescent Psychiatry who published it, and I expect when I get to that part of the story, the commenter will be well pleased with what I have to say. But, for the moment, I am stuck on the piece that is, to me, the most fundamental lesson from this story, and unfortunately many similar stories in the industry-funded clinical trial literature that fills our journals. The authors of this study did not function as physicians, clinicians, scientists, or even authors. Some were there because of their previous credentials. Others were there to build up future credentials. But they weren’t there to do what they presented themselves as doing. Researchers are practicing medicine too. I might be seen as naive to see this as the center of this story. If that’s the case, I’ll go to my grave being naive. Medicine has been my life, and this isn’t Medicine.

Some links that may be relevant to your post:
I think one needs to be careful to avoid possible caricatures of the primary investigators involvement:
(1993) http://dida.library.ucsf.edu/tid/bou38h10
(1993) http://dida.library.ucsf.edu/tid/cou38h10
(1995) http://dida.library.ucsf.edu/tid/hvu38h10
This would suggest your assessment of the level of interaction of the primary investigators with the patients themselves may be largely accurate:
(1994) http://dida.library.ucsf.edu/tid/qou38h10
Your characterization may not be entirely fair towards Drs. Klein and Geller:
(1999) http://dida.library.ucsf.edu/tid/lpu38h10
(1999) http://dida.library.ucsf.edu/tid/ppu38h10
But more fundamentally. You’re right in what I’m encouraging, but let me make clearer why I’m encouraging it, because I think it dovetails with your point:
You may be right in what you regard as the most fundamental point. But there is also the question of who is most likely to want to listen and care about any points made. And what will come from it. I keep suggesting focusing on JAACAP/AACAP precisely because it IS an organization where clinician physicians probably do outnumber these KOLs. Where their grass roots might care about these points. Banging the drum for years has done nothing to fundamentally impact individuals who keep bringing in money to universities, Grassley’s protestations notwithstanding.
I’m posting to your blog because I think that some who attack Study 329 also feel that most scientists and clinicians who work with kids are doing more harm than good. I do not believe that is the case for you. I believe it angers you this much for just the opposite reason. That the child psychiatrists and pediatricians and everyone else prescribing out there to kids deserve better that an abstract and a paper like this one.
If JAACAP required that for any paper they published the authors would have to sign an agreement to make all the related raw data public within say 5 years of publication that would be a start. If they broke that agreement they would be barred from publishing in JAACAP in the future. That would protect a drug company’s proprietary interest and would allow the researchers time to publish papers based on the time they had put into collecting it. It wouldn’t be perfect. But it would be a principled start. And it would have gone a very long way towards averting what happened in Study 329. Because the fears that Keller laid out in his 2001 email. At the very least they would have been forced to keep shopping for a journal that would have had less impact.
There is nothing I see in AACAP’s by laws that suggest the assembly and council could not vote on such a proposal at their upcoming meeting in October.
Throw a party for Dulcan and Keller and Ryan and Wagner for all anyone would care. The seeing of the mistakes is only to serve something good coming from it. That’s the purpose of all the comments. It’s not so much “justice” but improvement that may best serve the families out there.
By God some real improvement should come out of all of this. I get the sense you desperately want to see some improvement come out of this. Perhaps I am naïve to think that the people who work in the front lines with the kids are the ones in the best position to make it happen. But it’s an action that AACAP can take unilaterally and one would doubt that many KOLs sit on something they might consider as mundane and unlikely to affect anything as an assembly or council.
You catalyze something even approximating that kind of a change at a major journal like JAACAP (there aren’t a lot of journals that could theoretically be as directly impacted by clinician membership, and it’s the major child psychiatry journal as they keep saying) and I wouldn’t just be well pleased, I’d drive down and kiss you.
You’re right in what I’m encouraging, but let me make clearer why I’m encouraging it, because I think it dovetails with your point:
You may be right in what you regard as the most fundamental point. But there is also the question of who is most likely to want to listen and care about any points made. And what will come from it. I keep suggesting focusing on JAACAP/AACAP precisely because it IS an organization where clinician physicians probably do outnumber these KOLs. Where their grass roots might care about these points. Banging the drum for years has done nothing to fundamentally impact individuals who keep bringing in money to universities, Grassley’s protestations notwithstanding.
I’m posting to your blog because I think that some who attack Study 329 also feel that most scientists and clinicians who work with kids are doing more harm than good. I do not believe that is the case for you. I believe it angers you this much for just the opposite reason. That the child psychiatrists and pediatricians and everyone else prescribing out there to kids deserve better that an abstract and a paper like this one.
If JAACAP required that for any paper they published the authors would have to sign an agreement to make all the related raw data public within say 5 years of publication that would be a start. If they broke that agreement they would be barred from publishing in JAACAP in the future. That would protect a drug company’s proprietary interest and would allow the researchers time to publish papers based on the time they had put into collecting it. It wouldn’t be perfect. But it would be a principled start. And it would have gone a very long way towards averting what happened in Study 329. Because the fears that Keller laid out in his 2001 email. At the very least they would have been forced to keep shopping for a journal that would have had less impact.
There is nothing I see in AACAP’s by laws that suggest the assembly and council could not vote on such a proposal at their upcoming meeting in October.
Throw a party for Dulcan and Keller and Ryan and Wagner for all anyone would care. The seeing of the mistakes is only to serve something good coming from it. That’s the purpose of all the comments. It’s not so much “justice” but improvement that may best serve the families out there.
By God some real improvement should come out of all of this. I get the sense you desperately want to see some improvement come out of this. Perhaps I am naïve to think that the people who work in the front lines with the kids are the ones in the best position to make it happen. But it’s an action that AACAP can take unilaterally and one would doubt that many KOLs sit on something they might consider as mundane and unlikely to affect anything as an assembly or council.
You catalyze something even approximating that kind of a change at a major journal like JAACAP (there aren’t a lot of journals that could theoretically be as directly impacted by clinician membership, and it’s the major child psychiatry journal as they keep saying) and I wouldn’t just be well pleased, I’d drive down and kiss you.
Huzzah! Right on!
Dr. Mickey, save a kiss for me!
This is an amazing and important series.
See my comment on it up to now here:
http://hcrenewal.blogspot.com/2012/08/continuing-dissection-of-infamous-study.html
The listings of members on AACAP’s site and on the regional AACAP grassroot site webpages vary so I’m not sure how you get an up to date list of current AACAP council and assembly members. But this is an example of a letter that could be sent:
Dear Dr. X,
This message is addressed to you in your role as an AACAP council and/or assembly member.
It is to request that you consider voting, at your upcoming annual meeting in October, on a proposal that we believe will help JAACAP best serve practicing child psychiatrists, as well as others who work directly with children to support their mental health.
Our request:
That the AACAP assembly and council vote to require that prior to the publication of any research study in JAACAP, all authors and holders of the rights to the proprietary raw study data sign an agreement to make, by a fixed time (e.g. 5 years of publication in JAACAP), all of that raw data available (with appropriate redaction to protect patient confidentiality) to JAACAP to be made available to the scientific community at large. That, should they fail to do then further submissions from any of the signatories would not be considered for publication in JAACAP until the original agreement was honored. The fixed time chosen to apply to all articles would be one determined to be long enough to be fair in light of the enormous cost (in time, money and energy) of conducting a research study, as well as in light of the authors and data holders having valid academic and commercial interests. A possible number would be somewhere between 5-10 years.
This proposal is based on three simple beliefs:
1. No supporter, no guardian, of the reliability and integrity of a scientific resource can ever be as effective and resilient as true transparency.
2. To be true, this transparency must be applied as close to the actual raw study data as is reasonably feasible.
3. The need for that integrity and reliability cannot be felt more keenly than by those who rely upon the results of that science to inform their treatment of children.
This is the impetus for our request:
As you may or may not be aware, GlaxoSmithKline recently entered into a 3 billion dollar settlement with the Department of Justice. One of the central exhibits of the DOJ’s case was a paper published in 2001 in JAACAP. Due to the court case, there was public access to what would have otherwise remained proprietary study data. As part of its complaint, DOJ noted that active treatment had failed to separate from placebo on every primary and secondary outcome measure established in the original protocol. Other secondary measures had later been added and not all of those showed failure to separate from placebo. The abstract then stated that the conclusion of the paper was that the treatment was effective. Serious questions have also been raised about the nature of the reporting in the results section of both outcomes measures and adverse effects. Whatever one’s views of this paper, it is certain that assessing it has been aided by public access to the original raw study data. It is also possible that the original abstract and paper might have differed had it been know from the start that the raw data would eventually be made publicly available. It has been more than 10 years since the publication of this paper in the journal, and were it not for the action of the court the highest likelihood is that this otherwise proprietary data would still not be available.
We propose that transparency of the data to the scientific community at large is a more resilient assurance regarding any scientific resource than any single individual or group can ever be.
Deferring this transparency would not alter the knowledge of those involved that the presentation in the abstract and paper will eventually have to bear the scrutiny of the community as a whole. At the same time such a delay negates the objection that valid academic and commercial needs would be adversely impacted.
This is the balance struck by the Freedom of Information Act that the United States employs for a similar purpose.
It maximizes the trust that can be accorded the results and conclusions in the abstracts and related papers that a journal publishes. While at the same time leaving the current submission, review, and publication process autonomous and unchanged.
There are 8000 practicing child psychiatrists in the US. There are tens of thousands more pediatricians, family practitioners, and other professionals working with the mental health needs of children who also access information in the journal.
Such trust is central to AACAP and JAACAP’s mission.
Do not create another consensus committee to create yet another series of documents on this topic. Do not merely reflect on the importance of what is being discussed.
Act.
JAACAP is the premier journal in child mental health. It can exert leverage few other journals serving children and/or mental health can match.
It has been 10 years that the issues related to this paper have been raised.
Act.
Consider and pass this proposal at your upcoming meeting.
As one of the few members of AACAP’s council and/or assembly you are in a unique and wonderful position to do a meaningful good.
Thank you for your time and consideration.
Yours,
Dr. Poses,
Thank you for posting the link to your site. I had missed your discussions of 329. Sorry for burying your post between my musings. Here is the link to your great earlier post on 329:
http://hcrenewal.blogspot.com/2012/07/giant-gsk-settlement-provides-reminder.html?m=1
The quotes about physician payments and entertainment are unsurprising but still saddening.
For an organization like AACAP to actually act. To actually act on TRUE transparency. To show that they can be a force for true change from within. I meant what I said that those assembly and council members have a wonderful opportunity to enact a meaningful change. 1BOM is correct that nothing can rival the power of access to the actual raw data. To make the community as a whole the protector of integrity. Even if one were to agree with all the conclusions of the 2001 abstract/paper I don’t understand the objection. This is why I think it is crucial to shift the debate away from retraction. The 2001 paper no longer has impact (AACAP’s own 2007 practice parameters with Dr. Wagner as a contributor does not even reference it!). Act for True Transparency!
Sorry. Again, thanks for the great summary post Dr. Poses.
I neglected to include this paragraph after the “impetus for our request” paragraph:
A readership is concerned about scholarship, not simply whether an issue rises to the level of scientific error or misconduct. The trust that the readership has in the level of scholarship of the abstracts and related papers in a journal should not need to rest solely on the shoulders of the editor in chief. True eventual data transparency allows a detailed enough later re-examination of data as to provide an additional reassurance. It is difficult to read the documents made public through the court proceedings and not sense the importance of this. The content of the JAACAP reviewer comments suggest the editor in chief at JAACAP at the time had apparently made the editorial decision to ignore reviewer feedback that spoke to most of the serious concerns later raised. Similar concerns had been raised by reviewers at JAMA as part of their earlier rejection of a similar version of the paper. Also through court proceedings, specifically the DOJ complaint, we learn that, after the earlier JAMA feedback: ‘Given the comments received, GSK and the lead author decided to revise the article and send it to what they called “a less demanding journal.”‘
Dr. Wagner is one of the national delegates from the Texas regional. Mmmm, have some doubt she would be a supporter of this proposal. Bad omen perhaps. Action What a pipe dream. Yet another consensus panel in 2013 I’m sure. Dr. Ryan was on the last one in 2008, along with Dr. DelBello and Virginia Anthony. Sorry for the multiple posts on the previous comments sections, I could never quite get the hang of how to get it to work properly. LOL, don’t know what that implies. Thanks for your graciousness during this airing of my thoughts. I still hope you send out something to the council and assembly but the chance that any of them will truly care about this enough to do anything truly meaningful seems remote. Maybe someday. Thanks again 1BOM and good luck to you. Thanks for your efforts.
Mikey, could you possibly PDF your 329 series and send to me please? Reading hard copy is easier on the eye for me… and much more relaxing… even when the subject matter makes ones blood boil.
Great work!
Postscript re AACAP attitude towards 329 and the resultant 2001 JAACAP paper:
It is difficult to carefully read pages 4 through 18 of the doj complaint without being concerned about a possible attrition of trust in the abstracts and summaries of work that comes out of JAACAP.
http://www.justice.gov/opa/documents/gsk/us-complaint.pdf
As the following email suggests, that issue of trust is important to providers in the field:
http://www.healthyskepticism.org/files/docs/gsk/paroxetine/study329/20040413KellertoRyanetal.pdf
It is not entirely clear that AACAP’s leadership has a culture that promotes reflection upon past error and true change moving forward.
JAACAP Today
2001 paper’s abstract could be green lighted again by an editor in chief at JAACAP in exactly the same form today as then.
Change in endpoints are to be specified in the paper, but if they are not a response is not specified as that is not listed under the scientific misconduct section. A careful reading of the rules on authorship suggest that the title page of authors and acknowledgements could be written in largely the same way today as in 2001.
The 2001 abstract and paper may or may not be an accurate indication of the level of scholarship that the journal enforces today. It is not clear that AACAP has concerns about engaging in a process truly different from that observed for the 2001 paper.
The full text of their current guide for authors is here:
http://www.jaacap.com/authorinfo
Virginia Anthony and Dr. Dulcan
Anthony’s Assessment of Dulcan’s Tenure as Editor in Chief
http://psycnet.apa.org/psycinfo/2007-18374-001
You are not the guardians of the journal …
http://www.healthyskepticism.org/files/docs/gsk/paroxetine/study329/Dulcan.pdf
DULCAN: Oh I don’t have any regrets about publishing at all. It generated all sorts of useful discussion which is the purpose of a scholarly journal.
http://news.bbc.co.uk/2/hi/programmes/panorama/6317137.stm
Virginia Anthony and The Executive And Consensus Committees
Five members of Council make up the Executive Committee (EC): The President, President-Elect, Secretary, Treasurer, and Chair of the Assembly. The Executive Director of AACAP sits on the Executive committee and the Council as an ex-officio member. The current Executive Director, Virginia Q. “Ginger” Anthony, has been in the position for over 30 years, and has been recently honored by the American Medical Association as the most outstanding Director of a specialty society. We have been incredibly fortunate to have such an outstanding Executive Director. The President, Executive Director and EC manage the AACAP day to day business in between meetings of Council.
http://www.aacap.org/cs/root/developmentor/understanding_how_aacap_works
Ginger is clear about roles and boundaries. The officers determine policy and planning (with staff input and expertise when appropriate) and the staff implements. The staff report to the executive director NOT to the members or officers. Ginger knows the issues and is secure enough to address leadership when neces-
sary for the sake of the AACAP and its mission.
http://www.aacap.org/galleries/aacap_news/virginia_anthony_exec_director_of_aacap_retires_after_39_years.pdf
These ex-employee comments may be grossly inaccurate, but came up as a link related to AACAP:
http://www.glassdoor.com/Reviews/AACAP-Reviews-E249478.htm
2008 Consensus Committees Organized on a Related Topic,
Including list of participants
http://www.aacap.org/cs/root/physicians_and_allied_professionals/guidelines_on_conflict_of_interest_for_child_and_adolescent_psychiatrists
http://www.aacap.org/galleries/transparency-portal/Council%20Approved%20COI%20Guidelines%20Researchers%201-30-09.pdf
Perhaps it is fair to expect more of the front line doctors themselves. Perhaps the grassroots membership would view the issue of trust, JAACAP, and the 2001 abstract (which is often all prescribers actually review firsthand) differently than the AACAP leadership. That trust undergirds AACAP’s ability for its message to be respected by the other groups, including prescribers and legislators, who look for guidance in mental health care of children.
It is hard to believe that AACAP membership will read that first portion of the DOJ complaint and not have a strong concern about what it says about maintaining trust in information derived from abstracts in JAACAP, particularly if the issues raised are not more substantively addressed than they have been to this point.
It is harder to believe that providers and legislators outside of AACAP will read that DOJ complaint and not have a strong concern.
And for completeness sake (I know this has been linked to previously), the DOJ complaint with the relevant portions on pages 4 through 18.
We are not just speaking of lack of effectiveness by FDA standards. Pages 4-10 and pages 14-16 are of particular relevance to JAACAP and the 2001 paper that was used by the authors and GSK to tout this “landmark” study.
Careful reading of those pages would seem of worth to anyone relying upon information derived from JAACAP abstracts and summaries derived from them:
http://www.justice.gov/opa/documents/gsk/us-complaint.pdf
Those end users can determine whether or not that information is relevant to their level of trust. Particularly in those instances where they have not had the opportunity to review the primary paper in detail themselves.
A critical point: This is not a situation of non-clinicians and non-researchers not appreciating the “art vs the science” of medicine. At the heart of this is the scholarship in the abstract in relation to the study. A doctor can go ahead and prescribe off-label. They can prescribe without positive studies. But the statement the journal abstract is making, and the statement that the physician presenter for GSK was making when referencing the publication in a peer-reviewed journal, was to Study 329. The results from Study 329. Read the abstract from Study 329 (the one thing you could look at as a non-subscriber/non-academician unless the court had made the article and original study raw data available). See what conclusions and concerns would have come to mind. See what you would have reasonably expected to not be a concern given this was from a top tier and demanding journal. Then read pages 4-18 in the doj complaint. Then keep in mind that were there a near replication of the situation seen in Study 329 and the resultant abstract and paper that there would be little to no consequence of concern to authors or sponsors ENFORCED today by JAACAP/AACAP since it does not fall under scientific error or misconduct and there is no enforcement of adhering stringently to updating clinical registry information. Nor is there any requirement this be applied to the abstract. Nor is, as mentioned, there any clear detailing of enforcement of consequences should that not be the case. And, unless there is a lawsuit, the raw data would likely still never become public. There is a level of art that we reasonably want to accord doctors as clinicians when it comes to clinical decisions about patients. Is this the level of art we wish to accord them as editors in chief when it comes to the representation of the findings in scientific studies that inform those decisions?
Review the links above (and in earlier posts). Then look on your own for statements made by AACAP/JAACAP in the last 10 years in response to concerns have been raised about the study and this paper. I’m talking about the scholarship. NOT the issue of SSRIs and their relative benefits and risks. Which one study cannot answer on its own. A journal can only be expected to help with the presentation of the study data, and discussion, relevant to that paper. Would you feel more trust in abstracts/papers that would come out of JAACAP in the future if the same process that was in place in 2001 is in place in the future. Or if there were something additional in place?
Sorry if it’s not clear, but the last 3 posts were aimed at a general reader, not 1bom.
From the “Disclosing All Drug Data” section of
http://www.ncbi.nlm.nih.gov/pmc/articles/PMC1395768/
Comparing the “positive” study 329 and the “negative” study 377. Reading this acts as a reminder of how much papers from a journal can come to be discussed on the basis of their purported conclusions. This article also contains a plea for more data transparency from someone who sees it as essential to the research, not just clinical, enterprise.
“Keeping what may be mistakes secret is enormously problematic, not just because doctors and patients need all of the information they can get to make informed decisions, but because the people trying to inform them desperately need the benefit of collective experience.”
Sadly, his earnest “but the bigger question Is how can 2 different clinical trials produce antithetical data?” posed in Medscape back in 2004, referencing the 2001 JAACAP paper, probably has a simpler answer than he suspects.
Now that we can see the raw data from 329, they probably weren’t antithetical in the first place.
Something the community can judge for itself by having the benefit of now having access to that data.
And, one might wonder what approach the paper’s ghostwriter Sally Linden might have taken to the first draft she submitted to Smith Kline Beecham (in the end quite close to the final draft), had she known that a set number of years after publication in JAACAP the raw study data would be made available to the scientific community at large.